V001581
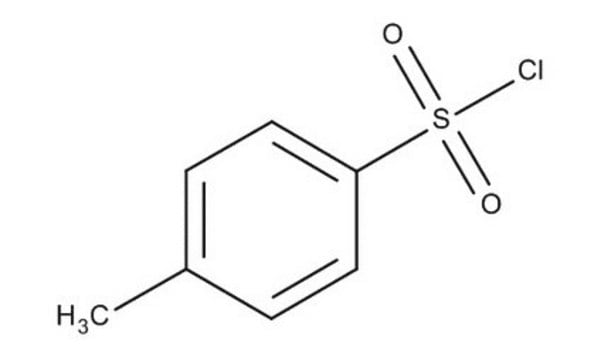
p-Toluenesulfonyl chloride
99%
Synonym(s):
Tosyl chloride
About This Item
Recommended Products
vapor pressure
1 mmHg ( 88 °C)
Quality Level
assay
99%
bp
134 °C/10 mmHg (lit.)
mp
65-69 °C (lit.)
SMILES string
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChI key
YYROPELSRYBVMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- An additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of aryl boronic acids in the absence of ligands.[2]
- A chlorine source for the α-chlorination of ketones in the presence of LDA.[3]
- A reactant in the tosylation of alcohols and phenols in the presence of heteropoly acids.[1]
- An activator for the reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.[4]
- A catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.[5]
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
262.4 °F - closed cup
flash_point_c
128 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service