PHL89593
Camptothecin
phyproof® Reference Substance
Synonym(s):
(S)-(+)-Camptothecin
About This Item
Recommended Products
grade
primary reference standard
product line
phyproof® Reference Substance
assay
≥90.0% (HPLC)
form
powder
manufacturer/tradename
PhytoLab
mp
260 °C (dec.) (lit.)
storage temp.
2-8°C
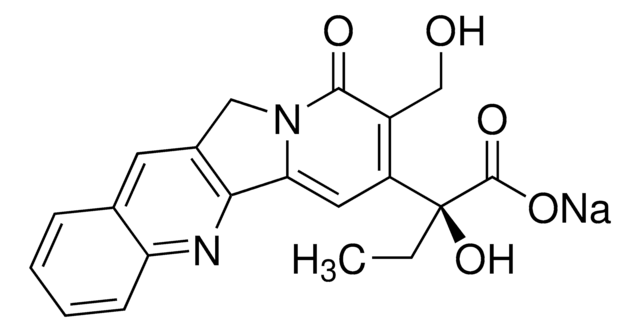
SMILES string
CC[C@@]1(O)C(=O)OCC2=C1C=C3N(Cc4cc5ccccc5nc34)C2=O
InChI
1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
InChI key
VSJKWCGYPAHWDS-FQEVSTJZSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Co-delivery system for camptothecin and doxorubicin: Research on dendritic polymer prodrug-based unimolecular micelles demonstrated a pH-responsive co-delivery mechanism for camptothecin and doxorubicin, offering a synergistic effect in controlled drug release (Chen and Liu, 2024).
- Investigation into camptothecin′s role in chronic myeloid leukemia: The study explored the therapeutic potential of FL118, a camptothecin derivative, against chronic myeloid leukemia resistant to BCR-ABL inhibitors, targeting RNA helicase DDX5 (Takeda et al., 2024).
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Muta. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service