This product has not been tested for solubility in DMSO, however various sources report that it is soluble at 20 mg/mL.
E1024
Estradiol
meets USP testing specifications
Synonym(s):
β-Estradiol, 1,3,5-Estratriene-3,17β-diol, 17β-Estradiol, 3,17β-Dihydroxy-1,3,5(10)-estratriene, Dihydrofolliculin
Select a Size
About This Item
Recommended Products
agency
USP/NF
meets USP testing specifications
Quality Level
form
powder
mp
176-180 °C (lit.)
application(s)
pharmaceutical (small molecule)
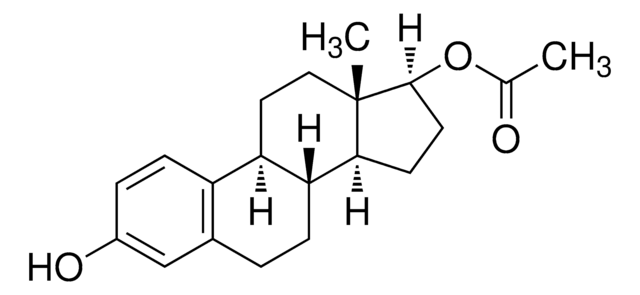
SMILES string
O[C@H]1CC[C@@]2([H])[C@]3([H])CCC4=CC(O)=CC=C4[C@@]3([H])CC[C@@]21C
InChI
1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1
InChI key
VOXZDWNPVJITMN-ZBRFXRBCSA-N
Gene Information
human ... ESR1(2099) , ESR2(2100) , ESRRB(2103) , GPER(2852) , SERPINA6(866)
mouse ... Esr1(13982) , Esr2(13983) , Esrra(26379)
rat ... Afp(24177) , Ar(24208) , Esr1(24890) , Esr2(25149) , Shbg(24775)
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Lact. - Repr. 1A
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
Is product E1024 soluble in DMSO? If so, what is the recommended initial stock concentration to make?
1 answer-
Helpful?
-
-
What is Product E1024, Estradiol, soluble in?
1 answer-
It is soluble in ethanol, acetone, dioxane, and sparingly in vegetable oils.
Helpful?
-
-
What is the solution stability of Product E1024, Estradiol?
1 answer-
Since this material is air sensitive, it is best to prepare solutions fresh.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the working range of Product E1024, Estradiol?
1 answer-
For cell culture applications, the working range of this compound is 0.2 - 10 ng/mL. For cell culture tested beta-estradiol, please refer to Sigma-Aldrich Prod. Nos. E2758 and E2257.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service