E0632
Enterokinase from porcine intestine
≥0.5 units/mg solid
Synonym(s):
Enteropeptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
salt-free, lyophilized powder
specific activity
≥0.5 units/mg solid
mol wt
150 kDa
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Enterokinase from porcine intestine has been used in a study to report a new experimental model of the anomalous pancreatico-biliary junction. Enterokinase from porcine intestine has also been used in a study to investigate the insulinotropic region of the gastric inhibitory polypeptide.
The enzyme from Sigma has been used for the activation of trypsinogen in order to measure the activity of trypsin in hog pancreas. The study showed that antimicrobial treatment reduces intestinal microflora and improves protein digestive capacity without changes in villous structure of weanling pigs.
Biochem/physiol Actions
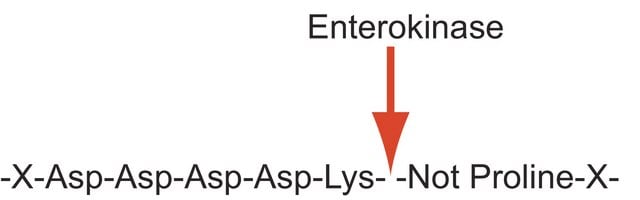
Enterokinase is a membrane bound serine protease that specifically and rapidly converts trypsinogen to trypsin, thereby, triggering the conversion of other zymogens to active enzymes. It has a molecular mass of approximately 150 kDa. The enzyme is a heterodimer consisting of 35-47 kDa subunits. The light and the heavy chains are linked by two disulfide bridges. It is a glycoprotein containing 35% carbohydrate. The polypeptide chain of trypsinogen is hydrolyzed only after an -(Asp)4-Lys- sequence. The enzyme is inhibited by soybean trypsin inhibitor. Enterokinase is typically used in protein modification and amino acid sequence determination.
Unit Definition
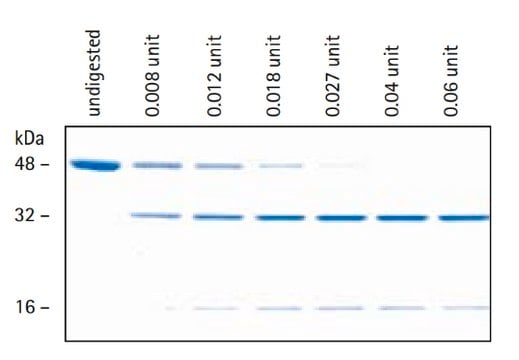
One unit will produce 1.0 nanomole of trypsin from trypsinogen per min at pH 5.6 at 25 °C.
substrate
Product No.
Description
Pricing
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas Thymann et al.
The British journal of nutrition, 97(6), 1128-1137 (2007-03-27)
The immediate post-weaning period is often associated with gut malfunction and diarrhoea for young pigs. Administration of antimicrobials remains an effective way to control weaning diarrhoea but it remains unclear how they affect gut physiology and microbiology although this is
Werner Hartwig et al.
Surgery, 144(3), 394-403 (2008-08-19)
A noninvasive model of necrohemorrhagic pancreatitis induced by simultaneous intravenous cerulein/enterokinase (EK) infusion has recently been established in rats. The aim of the present study was to establish this new model in mice and to compare it with the rat
T Benhidjeb et al.
Journal of pediatric surgery, 31(12), 1670-1674 (1996-12-01)
A model of anomalous pancreatico-biliary junction was developed and used to investigate a possible role in the development of choledochal cyst and tumors of the biliary tract. An anastomosis was constructed between an isolated pancreas-duodenal segment and the gallbladder in
Complementary DNA cloning and sequencing of rat enteropeptidase and tissue distribution of its mRNA.
N Yahagi et al.
Biochemical and biophysical research communications, 219(3), 806-812 (1996-02-27)
A cDNA clone encoding enteropeptidase (EC 3.4.21.9), a key enzyme for the conversion of trypsinogen to trypsin, was isolated from a rat duodenal mucosa cDNA library. Sequences of the 3585 base pair clone predicted that enteropeptidase is synthesized as a
V M Gabert et al.
International journal of pancreatology : official journal of the International Association of Pancreatology, 22(1), 39-43 (1997-08-01)
The results of this study demonstrated that proteolytic enzymes in pancreatic juice from pigs prepared with the pouch method (PM) were nearly fully active or were fully active. When activation with enterokinase was carried out further inactivation and/or breakdown occurred
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service