D5018
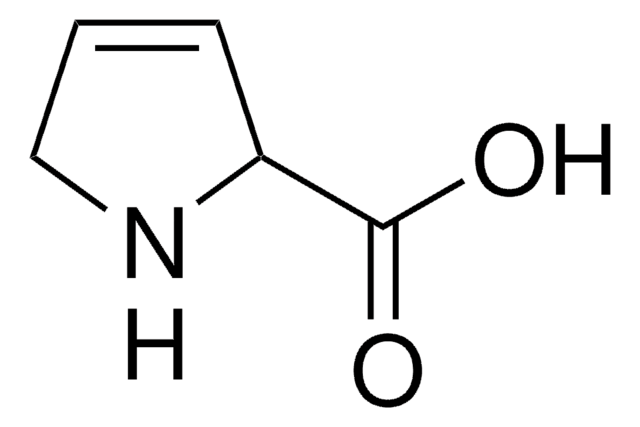
3,4-Dehydro-L-proline methyl ester hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H9NO2 · HCl
CAS Number:
Molecular Weight:
163.60
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
form
solid
storage temp.
−20°C
SMILES string
Cl.COC(=O)C1NCC=C1
InChI
1S/C6H9NO2.ClH/c1-9-6(8)5-3-2-4-7-5;/h2-3,5,7H,4H2,1H3;1H
InChI key
LDWTUXKKNIHFQG-UHFFFAOYSA-N
Biochem/physiol Actions
3,4-Dehydro-L-proline methyl ester is C-terminal blocked 3,4-Dehydro-L-proline (3,4-DHP). 3,4-Dehydro-L-proline is used as a substrate and inhibitor of various enzymes. 3,4-Dehydro-L-proline may be used to inhibit extensin biosynthesis. 3,4-Dehydro-L-proline is a alternate substrate of the amino acid oxidase, NikD. 3,4-Dehydro-L-proline inhibits collagen secretion by chondorcytes.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA).
Xu C, Takac T, Burbach C, et al.
BMC plant biology, 24, 38-38 (2011)
Hyon Jong Kim et al.
The Journal of biological chemistry, 283(16), 10310-10317 (2008-02-19)
Physiological mineralization in growth plate cartilage is highly regulated and restricted to terminally differentiated chondrocytes. Because mineralization occurs in the extracellular matrix, we asked whether major extracellular matrix components (collagens) of growth plate cartilage are directly involved in regulating the
Annick Méjean et al.
Biochemistry, 49(1), 103-113 (2009-12-04)
Anatoxin-a and homoanatoxin-a are two potent cyanobacterial neurotoxins. We recently reported the identification of the gene cluster responsible for the biosynthesis of these toxins in cyanobacteria and proposed a biosynthetic scheme starting from L-proline and involving three polyketide synthases for
Phaneeswara-Rao Kommoju et al.
Biochemistry, 48(40), 9542-9555 (2009-08-26)
NikD is a flavoprotein oxidase that catalyzes the oxidation of piperideine-2-carboxylate (P2C) to picolinate in a remarkable aromatization reaction comprising two redox cycles and at least one isomerization step. Tyr258 forms part of an "aromatic cage" that surrounds the ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service