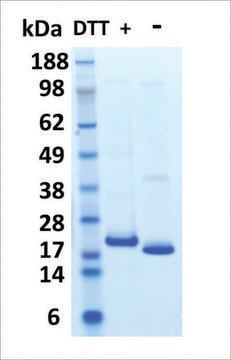
S0947000
Somatropin
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
somatropin
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Somatropin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Related product
Product No.
Description
Pricing
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Editor's commentary: sex hormone priming.
Zvi Laron
Pediatric endocrinology reviews : PER, 11(3), 288-288 (2014-04-11)
Z Gucev et al.
Pediatric endocrinology reviews : PER, 11(3), 337-338 (2014-04-11)
The Second meeting on Rare Diseases in South Eastern Europe (SEE) was held in Skope, Macedonia on November 15-16, 2013. Objective and main data: Rare diseases (RD) are a major problem in developed and especially in countries without affluence. 6-8%
Marianne Klose et al.
The Journal of clinical endocrinology and metabolism, 99(1), 101-110 (2013-11-19)
Recent international guidelines suggest pituitary screening in patients with moderate and severe traumatic brain injury (TBI). Predominantly isolated GH deficiency (GHD) was reported in the literature, raising the question of potential methodological bias. Our objective was to assess the prevalence
Long-acting growth hormone.
Charlotte Höybye et al.
Paediatric drugs, 15(6), 427-429 (2013-11-20)
Vita Birzniece et al.
European journal of endocrinology, 170(3), 375-383 (2013-12-19)
GH action is attenuated by estrogens and selective estrogen receptor modulators (SERMs) administered orally. During GH therapy in hypopituitary women, co-treatment with raloxifene, a SERM, induced a smaller gain in lean body mass (LBM) compared with estrogen, despite an equal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service