PHR1055
Famotidine
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
{3-[((2-((Aminoiminomethyl)amino)-4-thiazolyl)methyl)thio]-N′-(aminosulfonyl)propaneimidamide}, N′-(Aminosulfonyl)-3-([2-(diaminomethyleneamino)-4-thiazolyl]methylthio)propanamidine
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
certified reference material
pharmaceutical secondary standard
agency
BP
EP
USP
traceable to BP 653
traceable to Ph. Eur. F0050000
traceable to USP 1269200
vapor pressure
<0.0000001 kPa ( 25 °C)
API family
famotidine
CofA
current certificate can be downloaded
packaging
pkg of 1 g
storage condition
protect from light (20 mm aluminium crimp seal for unused portion)
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
color
white to pale yellow-white
mp
325.4-327.2 °F (163—164°C)
solubility
DMF: freely soluble
acetone: practically insoluble
chloroform: practically insoluble
ethanol: practically insoluble
ether: practically insoluble
ethyl acetate: practically insoluble
glacial acetic acid: freely soluble
methanol: slightly soluble
water: very slightly soluble
application(s)
pharmaceutical (small molecule)
format
neat
shipped in
ambient
storage temp.
2-8°C
SMILES string
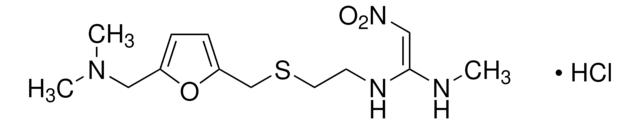
N\C(N)=N\c1nc(CSCCC(=N)NS(N)(=O)=O)cs1
InChI
1S/C8H15N7O2S3/c9-6(15-20(12,16)17)1-2-18-3-5-4-19-8(13-5)14-7(10)11/h4H,1-3H2,(H2,9,15)(H2,12,16,17)(H4,10,11,13,14)
InChI key
XUFQPHANEAPEMJ-UHFFFAOYSA-N
Gene Information
human ... HRH2(3274)
Looking for similar products? Visit Product Comparison Guide
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Application
Biochem/physiol Actions
Analysis Note
Other Notes
Footnote
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service