H7753
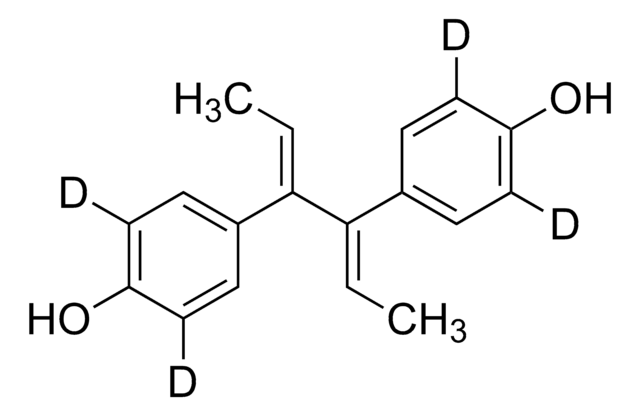
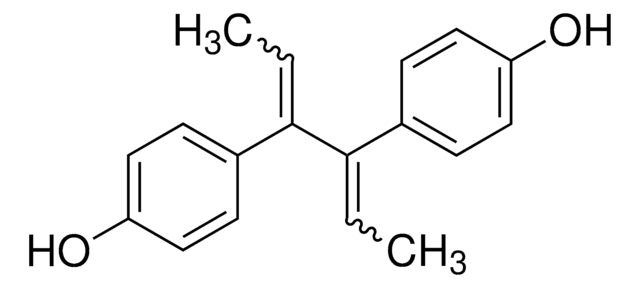
Hexestrol
analytical standard
Synonym(s):
4,4′-(1,2-Diethylethylene)diphenol, meso-3,4-Bis(4-hydroxyphenyl)hexane, Dihydrodiethylstilbestrol
About This Item
Recommended Products
grade
analytical standard
Quality Level
assay
≥98%
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
forensics and toxicology
pharmaceutical (small molecule)
veterinary
format
neat
SMILES string
CC[C@H]([C@H](CC)c1ccc(O)cc1)c2ccc(O)cc2
InChI
1S/C18H22O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,17-20H,3-4H2,1-2H3/t17-,18+
InChI key
PBBGSZCBWVPOOL-HDICACEKSA-N
Gene Information
human ... ESR1(2099)
rat ... Esr1(24890)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Linkage
Recommended products
signalword
Danger
hcodes
Hazard Classifications
Carc. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service