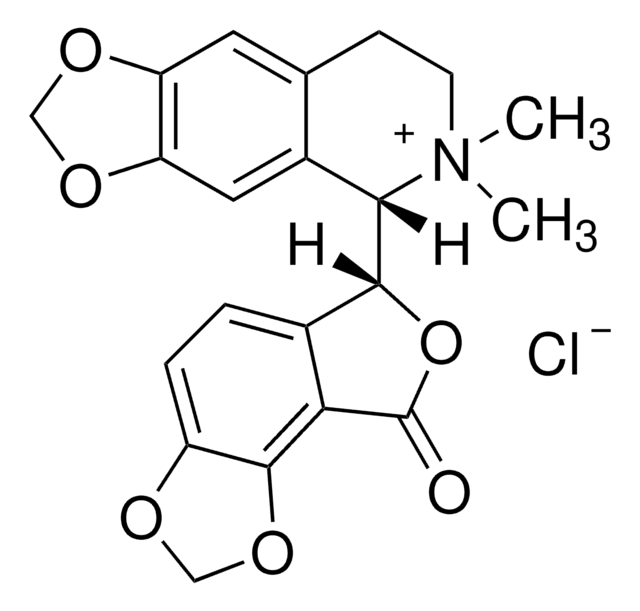
14340
(+)-Bicuculline
≥97.0% (TLC)
Synonym(s):
Bucuculline
Select a Size
About This Item
Recommended Products
Quality Level
assay
≥97.0% (TLC)
form
powder
optical activity
[α]20/D +126±6°, c = 1% in chloroform
mp
193-197 °C
SMILES string
[H][C@]1(OC(=O)c2c3OCOc3ccc12)[C@@]4([H])N(C)CCc5cc6OCOc6cc45
InChI
1S/C20H17NO6/c1-21-5-4-10-6-14-15(25-8-24-14)7-12(10)17(21)18-11-2-3-13-19(26-9-23-13)16(11)20(22)27-18/h2-3,6-7,17-18H,4-5,8-9H2,1H3/t17-,18+/m0/s1
InChI key
IYGYMKDQCDOMRE-ZWKOTPCHSA-N
Gene Information
rat ... Gabra2(29706)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a compound to compare pharmacodynamics and network activity profiles of conolidine/cannabidiol[3]
- to study the effects of chronic caffeine administration on the function of GABAA receptor[4]
- to isolate N-methyl-D-aspartate receptor (NMDAR)-specific evoked and miniature excitatory postsynaptic currents (eEPSCs and mEPSCs) in neurons of rats[5]
Biochem/physiol Actions
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Yeast is one of the most important microorganisms known and utilised by mankind. Ancient Middle Eastern civilisations used the organism to bake bread and to produce mead, beer and wine.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service