12070
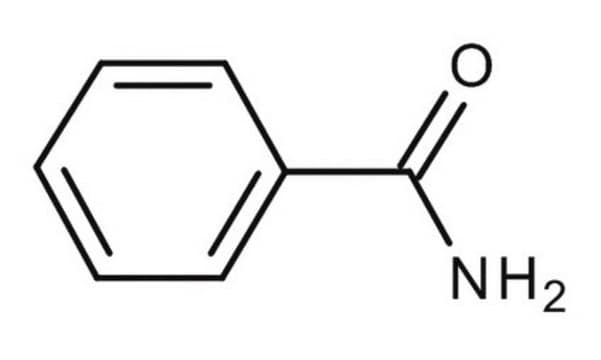
Benzamide
purum, ≥98.0% (HPLC)
Synonym(s):
Benzoic acid amide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CONH2
CAS Number:
Molecular Weight:
121.14
Beilstein/REAXYS Number:
385876
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
grade
purum
assay
≥98.0% (HPLC)
mp
125-128 °C (lit.)
125-128 °C
solubility
methanol: soluble 1 g/10 mL, clear, colorless to faintly yellow
SMILES string
NC(=O)c1ccccc1
InChI
1S/C7H7NO/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H2,8,9)
InChI key
KXDAEFPNCMNJSK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Benzamide was used to study the effect of inhibitors of endonuclease and poly(ADP ribose) polymerase on anti-Fas monoclonal antibody treated Jurkat cells.
Biochem/physiol Actions
Benzamide is a specific inhibitor of poly(ADP-ribose)polymerase. It prevents glutamate- and methamphetamine-induced neurotoxicity in vitro in C57B1/6N mouse.
Inhibits poly(ADP-ribose) polymerase (PARP).
replaced by
Product No.
Description
Pricing
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Muta. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
356.0 °F - closed cup
flash_point_c
180 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
I Yamadori et al.
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 46(1), 85-90 (1997-12-24)
We compared two methods to stain apoptotic cells, one using terminal deoxynucleotidyl transferase (TDT), the other DNA polymerase I, using leukemia cell lines treated with anti-Fas monoclonal antibody (MAb). Both TDT and polymerase I strongly reacted with fragmented nuclei of
E Kun et al.
Proceedings of the National Academy of Sciences of the United States of America, 80(23), 7219-7223 (1983-12-01)
Human fibroblasts were subjected to nutritionally induced G1 block, followed by release and subsequent entry into S phase, and exposed to nontoxic concentrations of carcinogens in early S phase. Cell transformation occurred as determined by early morphologic cell alterations, anchorage-independent
C Cosi et al.
Brain research, 735(2), 343-348 (1996-10-07)
Previous studies have indicated that the activation of poly(ADP-ribose) polymerase (PARP), an enzyme involved in DNA plasticity-related phenomena, is an early event occurring in glutamate-induced neurotoxicity in vitro, and that inhibitors of PARP, including benzamide, are protective against both glutamate-
James M Wright et al.
The Cochrane database of systematic reviews, 4, CD001841-CD001841 (2018-04-19)
This is the first update of a review published in 2009. Sustained moderate to severe elevations in resting blood pressure leads to a critically important clinical question: What class of drug to use first-line? This review attempted to answer that
J D Coyle et al.
Journal of pharmaceutical sciences, 76(5), 402-405 (1987-05-01)
We report a reversed-phase high-performance liquid chromatography method for the determination of procainamide (PA) and three of its metabolites, n-acetylprocainamide (NAPA), deethylprocainamide (DEPA), and deethyl-n-acetylprocainamide (DENAPA), in serum and urine. (p-Amino)-n-(2-dipropylaminoethyl)-benzamide was the internal standard. A phenyl column (1.0-mL/min flow
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service