E464
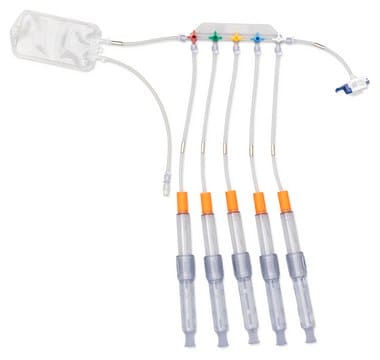
NovaSeptum® GO Accurate Volume Syringe Sampling System, Manifold (5-valve)
sterile; β-irradiated
Synonym(s):
NovaSeptum® GO Accurate Volume Syringes
About This Item
Recommended Products
material
316 stainless steel cannula
PureFlex™ polyethylene bag (Drainage bag)
polycarbonate syringe (fluid contact layer)
polyester body
silicone septum (platinum-cured)
silicone syringe (medical fluid contact layer; platinum cured silicone)
silicone tubing
Quality Level
agency
according to ISO 11137 (sterilization)
according to ISO 146441
meets USP 88 biological reactivity (all component materials)
sterility
sterile; β-irradiated
product line
NovaSeptum® GO
feature
autoclavable: no
parameter
-20-50 °C temp. range (-4-122 °F)
0.50 bar max. pressure (7.25 psi)
impurities
<2.15 EU/device bacterial endotoxins (LAL test)
fitting
female Luer-Lok® outlet
male Luer-Lok® outlet
application(s)
cell therapy
gene therapy
life science and biopharma
mAb
ophthalmics
parenterals
pathogen testing
pathogen testing
pharma/biopharma processes
pyrogen testing
sterile sampling
sterility testing
viral therapy
storage temp.
room temp
Looking for similar products? Visit Product Comparison Guide
General description
NovaSeptum® GO Accurate Volume Syringes are designed to take samples from 1 mL to 20 mL, enabling operators to directly dispense sample products with milliliter accuracy. The ability to accurately measure the sample taken is particularly important for sampling of high value products and reduces expensive product waste. The syringes are ideal for storage. A closed, sterile sampling method can significantly reduce risk of cross contamination. Ideal for sampling from aseptic and sterile processes, the NovaSeptum® GO sterile sampling system provides consistently representative samples, safely and securely.
Application
Packaging
E464-90020 (5 x 20 ml syringes, 5 units per box)
Components
- Septum: Platinum-cured silicone
- Body: Polyester
- Cannula: ASTM® 316 L Stainless steel
- Drainage bag: Polyethylene film (PureFlex™ film)
- Fluid contact layer (syringe): Polycarbonate, platinum-cured silicone, medical silicone fluid
- Tubing: Silicone
- Outlet Tubing: Female and male Luer-Lok®
Preparation Note
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This article explains how to streamline transfer of an allogeneic cell therapies process to a CDMO partner and long-term manufacturing success.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service