8.02954
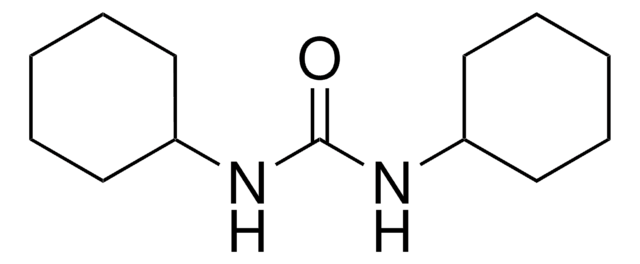
N,N′-Dicyclohexylcarbodiimide
for peptide synthesis
Synonym(s):
N,N′-Dicyclohexylcarbodiimide, DCC
About This Item
Recommended Products
product name
N,N′-Dicyclohexylcarbodiimide, for synthesis
Quality Level
form
solid
potency
1110 mg/kg LD50, oral (Rat)
71 mg/kg LD50, skin (Rat)
reaction suitability
reaction type: Coupling Reactions
bp
148-152 °C/15 hPa
mp
35-36 °C
transition temp
flash point 113 °C
density
0.95 g/cm3 at 40 °C
bulk density
920 kg/m3
application(s)
peptide synthesis
storage temp.
2-30°C
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
InChI key
QOSSAOTZNIDXMA-UHFFFAOYSA-N
Related Categories
General description
Application
- The synthesis of optically pure N-acyl-N,N′-dicyclohexylureas.
- The activation of the carboxylic acid groups in aromatic carboxylic acids to facilitates their reaction with (N-isocyanimino)trifluoroacetamide to form the corresponding 1,3,4-oxadiazole derivatives.
- The synthesis of poly (vinyl alcohol-co-vinyl levulinate) copolymers for use in biomedical applications.
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Storage Class
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service