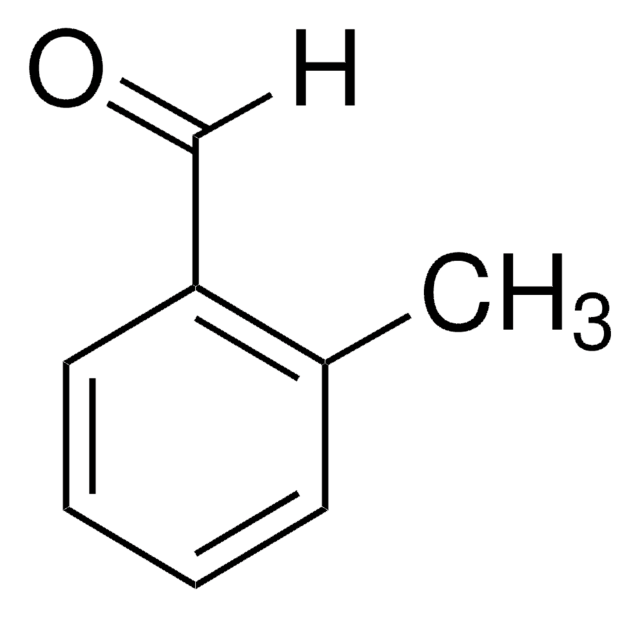
T35602
p-Tolualdehyde
97%
Synonym(s):
4-Methylbenzaldehyde
About This Item
Recommended Products
assay
97%
refractive index
n20/D 1.545 (lit.)
bp
204-205 °C (lit.)
82-85 °C/11 mmHg (lit.)
solubility
water: soluble 0.25 g/L at 25 °C
density
1.019 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)c1ccc(C)cc1
InChI
1S/C8H8O/c1-7-2-4-8(6-9)5-3-7/h2-6H,1H3
InChI key
FXLOVSHXALFLKQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
161.6 °F - closed cup
flash_point_c
72 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service