B92001
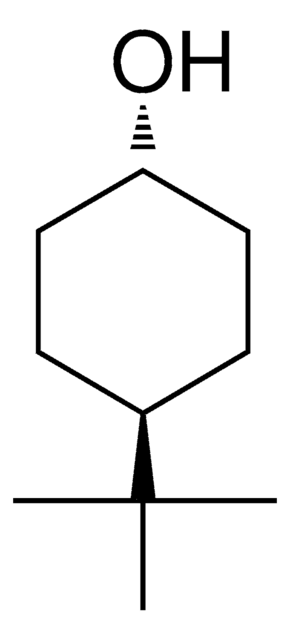
4-tert-Butylcyclohexanol, mixture of cis and trans
98%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CC6H10OH
CAS Number:
Molecular Weight:
156.27
Beilstein/REAXYS Number:
1902277
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
powder
bp
110-115 °C/15 mmHg (lit.)
mp
62-70 °C (lit.)
SMILES string
CC(C)(C)C1CCC(O)CC1
InChI
1S/C10H20O/c1-10(2,3)8-4-6-9(11)7-5-8/h8-9,11H,4-7H2,1-3H3
InChI key
CCOQPGVQAWPUPE-UHFFFAOYSA-N
Application
4-tert-Butylcyclohexanol (mixture of cis and trans) can be used as a reactant to synthesize tris(4,4′-di-tert-butyl-2,2′-bipyridine)(trans-4-tert-butylcyclohexanolato)deca-μ-oxido-heptaoxidoheptavanadium oxide cluster complex by reacting with [V8O20(C18H24N2)4]. It can also be used as a reactant in competitive Oppenauer oxidation experiments in the presence of zeolite BEA as a stereoselective catalyst. Only cis-isomer is selectively converted to the corresponding ketone, whereas trans-isomer remains unchanged.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
221.0 °F - closed cup
flash_point_c
105 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tris (4, 4?-di-tert-butyl-2, 2?-bipyridine)(trans-4-tert-butylcyclohexanolato) deca- ? -oxido-heptaoxidoheptavanadium acetonitrile monosolvate including another unknown solvent molecule
Kodama S, et al.
IUCrData, 5(4), x200449-x200449 (2020)
Stereoselective Meerwein-Ponndorf-Verley and Oppenauer reactions catalysed by zeolite BEA
Creyghton EJ, et al.
J. Mol. Catal. A: Chem., 115(3), 457-472 (1997)
Mireia Oromí-Farrús et al.
Journal of analytical methods in chemistry, 2012, 452949-452949 (2012-06-01)
The use of iodine as a catalyst and either acetic or trifluoroacetic acid as a derivatizing reagent for determining the enantiomeric composition of acyclic and cyclic aliphatic chiral alcohols was investigated. Optimal conditions were selected according to the molar ratio
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service