All Photos(1)
About This Item
Empirical Formula (Hill Notation):
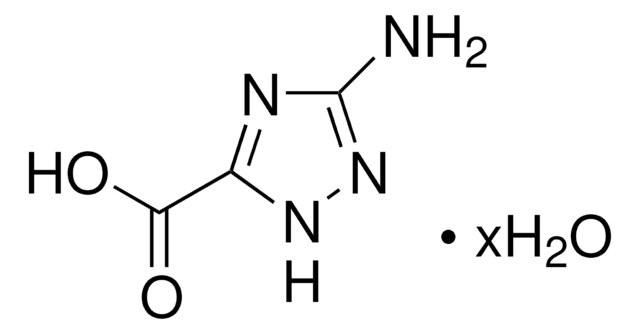
C5H5N3O2
CAS Number:
Molecular Weight:
139.11
Beilstein/REAXYS Number:
124835
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥99%
form
powder
mp
205-210 °C (dec.) (lit.)
SMILES string
Nc1nccnc1C(O)=O
InChI
1S/C5H5N3O2/c6-4-3(5(9)10)7-1-2-8-4/h1-2H,(H2,6,8)(H,9,10)
InChI key
ZAGZIOYVEIDDJA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 52 ( Pt 6), 1512-1514 (1996-06-15)
3-Aminopyrazine-2-carboxylic acid, C5H5N3O2, displays an extensive network of intra- and intermolecular hydrogen bonds which are undoubtedly responsible for the modest values of the displacement parameters. H-atom transfer to the ring N atoms did not occur and the carboxy and amino
Ghada Bouz et al.
Molecules (Basel, Switzerland), 24(7) (2019-03-31)
We report the design, synthesis, and in vitro antimicrobial activity of a series of N-substituted 3-aminopyrazine-2-carboxamides with free amino groups in position 3 on the pyrazine ring. Based on various substituents on the carboxamidic moiety, the series is subdivided into
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service