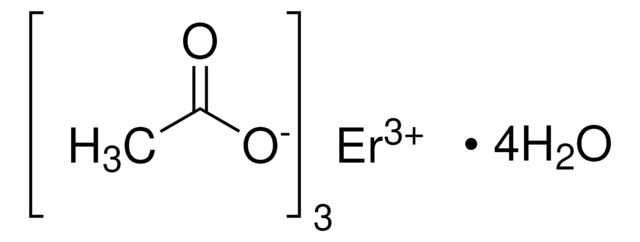
930962
Yttrium(III) acetate tetrahydrate
99.99% trace rare earth metals basis
Synonym(s):
Acetic acid yttrium(3+) salt, Yttrium triacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
assay
99.99% trace rare earth metals basis
form
powder
impurities
≤150 ppm trace rare earth metals
≤500 ppm trace metals
mp
350 °C (Decomp.)
solubility
H2O: soluble (lit.)
density
1.5 g/cm3
General description
Yttrium acetate tetrahydrate is a white, crystalline salt. The salt is soluble in water and mineral acids as well as solutions, such as methoxyethanol with diethylenetriamine, that complex with the Y3+ cations.
Application
Yttrium acetate is a common reactant in the synthesis of yttrium compounds including yttrium oxides and yttrium fluorides. Yttrium acetate is particularly useful because of its solubility in and low thermal decomposition temperature, which make it attractive for hydrothermal reactions and co-precipitation processing before calcination.
A major application of high-purity yttrium acetate is in the synthesis of sodium yttrium fluoride (NaYF4) nanoparticles. Typically, in these syntheses, yttrium acetate is mixed with oleic acid in octadecene and heated to form Y(oleate)3, which is reacted with ammonium fluoride and sodium hydroxide in methanol at modest temperatures (e.g. 50 C) to form NaYF4 nanoparticles. This synthesis offers great control over particle size and crystallinity and allows for easy incorporation rare-earth metal dopants.
Lanthanide-doped NaYF4 nanoparticles are one of the most studied materials for up conversion. These nanoparticles, which can convert two photons of near-infrared (NIR) light into visible light, have important in-vivo applications because of the deep tissue penetration abilities of NIR. For example, these nanoparticles have been used for in-vivo Zn2+ optical sensing, in-vivo ratiometric sensing of lymphatic inflammation,, and in-vivo sensing of peroxynitrite.
A major application of high-purity yttrium acetate is in the synthesis of sodium yttrium fluoride (NaYF4) nanoparticles. Typically, in these syntheses, yttrium acetate is mixed with oleic acid in octadecene and heated to form Y(oleate)3, which is reacted with ammonium fluoride and sodium hydroxide in methanol at modest temperatures (e.g. 50 C) to form NaYF4 nanoparticles. This synthesis offers great control over particle size and crystallinity and allows for easy incorporation rare-earth metal dopants.
Lanthanide-doped NaYF4 nanoparticles are one of the most studied materials for up conversion. These nanoparticles, which can convert two photons of near-infrared (NIR) light into visible light, have important in-vivo applications because of the deep tissue penetration abilities of NIR. For example, these nanoparticles have been used for in-vivo Zn2+ optical sensing, in-vivo ratiometric sensing of lymphatic inflammation,, and in-vivo sensing of peroxynitrite.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Juanjuan Peng et al.
Angewandte Chemie (International ed. in English), 56(15), 4165-4169 (2017-03-16)
Drug toxicity is a long-standing concern of modern medicine. A typical anti-pain/fever drug paracetamol often causes hepatotoxicity due to peroxynitrite ONOO- . Conventional blood tests fail to offer real-time unambiguous visualization of such hepatotoxicity in vivo. Here we report a luminescent
Shangfeng Wang et al.
Nano letters, 19(4), 2418-2427 (2019-03-19)
Quantitatively imaging the spatiotemporal distribution of biological events in living organisms is essential to understand fundamental biological processes. Self-calibrating ratiometric fluorescent probes enable accurate and reliable imaging and sensing, but conventional probes using wavelength of 400-900 nm suffer from extremely
Hard Proof of the NaYF4/NaGdF4 Nanocrystal Core/Shell Structure.
Abel K A, et al.
Journal of the American Chemical Society, 131, 14644-14645 (2009)
Zhenglin Yang et al.
Journal of the American Chemical Society, 140(50), 17656-17665 (2018-11-15)
Spatial and temporal distributions of metal ions in vitro and in vivo are crucial in our understanding of the roles of metal ions in biological systems, and yet there is a very limited number of methods to probe metal ions
Two-Photon Upconversion Laser (Scanning and Wide-Field) Microscopy Using Ln3+-Doped NaYF4 Upconverting Nanocrystals: A Critical Evaluation of their Performance and Potential in Bioimaging.
Pichaandi J, et al.
The Journal of Physical Chemistry C, 115, 19054-19064 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service