803928
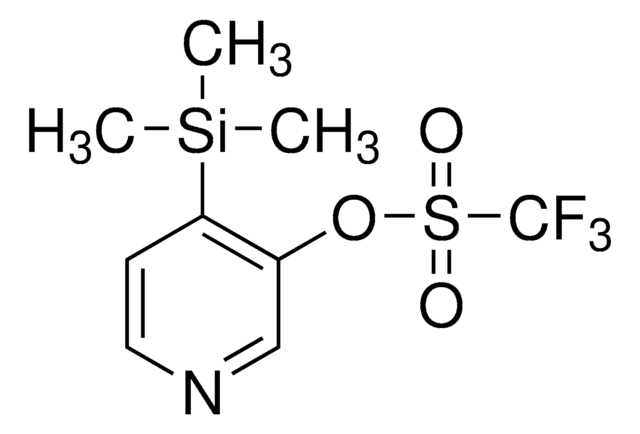
Benzyl 4-(trifluoromethylsulfonyloxy)-3-(trimethylsilyl)-5,6-dihydropyridine-1(2H)-carboxylate
Synonym(s):
Garg Azacyclohexyne Precursor
About This Item
Recommended Products
description
FP: >230°F
form
liquid
refractive index
n20/D 1.4902
density
1.2625 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
C[Si](C)(C)C1=C(OS(=O)(C(F)(F)F)=O)CCN(C(OCC2=CC=CC=C2)=O)C1
InChI
1S/C17H22F3NO5SSi/c1-28(2,3)15-11-21(16(22)25-12-13-7-5-4-6-8-13)10-9-14(15)26-27(23,24)17(18,19)20/h4-8H,9-12H2,1-3H3
InChI key
QGKRRFLQHRMSAL-UHFFFAOYSA-N
General description
application
related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Garg group develops methods for the synthesis of natural products and pharmaceuticals. One key method pertains to heterocyclic arynes, such as indolynes and pyridynes, which are generated in situ from silyltriflate precursors under mild fluoride based reaction conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service