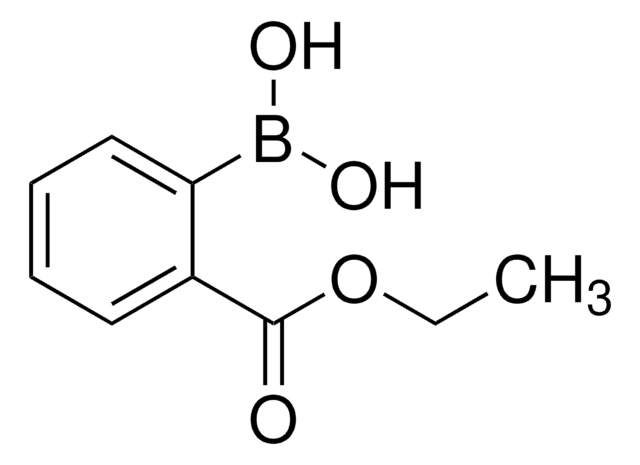
594539
4-Methoxycarbonylphenylboronic acid
≥95%
Synonym(s):
(4-Carbomethoxyphenyl)boronic acid, 4-Carbomethoxybenzeneboronic acid, 4-Methoxycarbonylbenzeneboronic acid, 4-borono-benzoic acid 1-methyl ester, p-(Methoxycarbonyl)boronic acid, p-(Methoxycarbonyl)phenylboronic acid, p-borono-benzoic acid methyl ester, Methyl 4-boronobenzoate, Methyl p-boronobenzoate
About This Item
Recommended Products
assay
≥95%
form
powder
mp
197-200 °C (lit.)
SMILES string
COC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO4/c1-13-8(10)6-2-4-7(5-3-6)9(11)12/h2-5,11-12H,1H3
InChI key
PQCXFUXRTRESBD-UHFFFAOYSA-N
Related Categories
Application
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- One-pot ipso-nitration of arylboronic acids
- Copper-catalyzed nitration
- Cyclocondensation followed by palladium-phosphine-catalyzed Suzuki-Miyaura coupling
- Reagent used in Preparation of
- Biaryls via nickel-catalyzed Suzuki-Miyaura cross-coupling reaction of aryl halides with arylboronic acid†
- Chromenones and their bradykinin B1 antagonistic activit†
- Pt nanoparticles@Photoactive metal-organic frameworks resulting in efficient hydrogen evolution via synergistic photoexcitation and electron injectio†
- Salicylate-based thienylbenzoic acids as E. coli methionine aminopeptidase inhibitor†
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)