All Photos(1)
About This Item
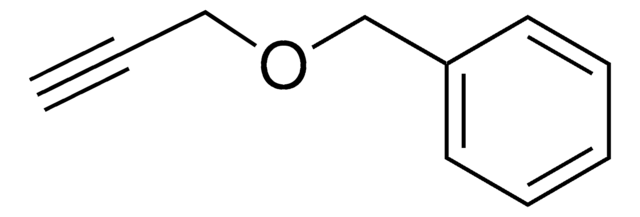
Linear Formula:
C6H5CO2CH2C≡CH
CAS Number:
Molecular Weight:
160.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
liquid
refractive index
n20/D 1.5320 (lit.)
bp
225-226 °C (lit.)
density
1.106 g/mL at 25 °C (lit.)
SMILES string
O=C(OCC#C)c1ccccc1
InChI
1S/C10H8O2/c1-2-8-12-10(11)9-6-4-3-5-7-9/h1,3-7H,8H2
InChI key
NBDHEMWCIUHARG-UHFFFAOYSA-N
Related Categories
General description
Propargyl benzoate is an aromatic ester containing a terminal acetylene group. It can be synthesized by reacting propargyl bromide and benzoyl chloride.
Application
Propargyl benzoate may be used in the preparation of amphiphilic graft copolymers of poly(ε-caprolactone) (PCL).
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Combination of ring-opening polymerization and ?click chemistry?: toward functionalization and grafting of poly (?-caprolactone).
Riva R, et al.
Macromolecules, 40(4), 796-803 (2007)
Nickel-Catalyzed Tandem Coupling of ?,?-Enones, Alkynes, and Alkynyltins for the Regio-and Stereoselective Synthesis of Conjugated Enynes.
Ikeda S, et al.
The Journal of Organic Chemistry, 61(23), 8248-8255 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service