All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H11N3
CAS Number:
Molecular Weight:
173.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
114-117 °C (lit.)
SMILES string
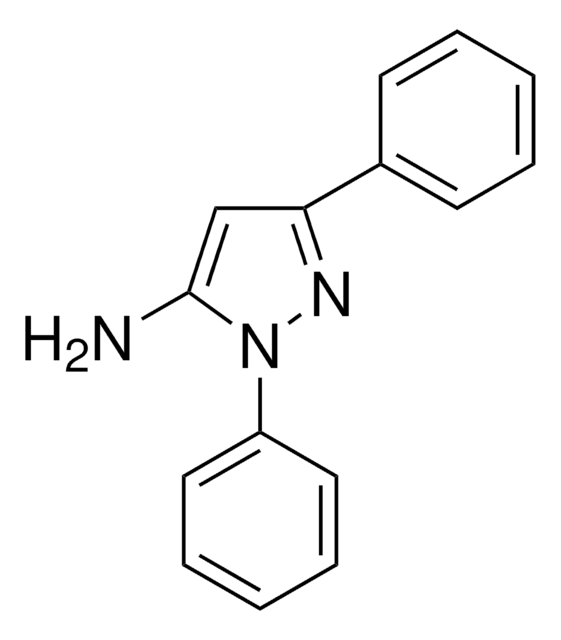
Cc1cc(N)n(n1)-c2ccccc2
InChI
1S/C10H11N3/c1-8-7-10(11)13(12-8)9-5-3-2-4-6-9/h2-7H,11H2,1H3
InChI key
FMKMKBLHMONXJM-UHFFFAOYSA-N
Related Categories
General description
5-Amino-3-methyl-1-phenylpyrazole is an aminopyrazole derivative. It reacts with 6-methyl-4-oxo-4H-[1]-benzopyran-3-carboxaldehyde to yield 5-(2-hydroxy-5-methylbenzoyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine and 2-methoxy-6-methyl-3-(3-methyl-1-phenylpyrazol-5-ylaminomethylene)chroman-4-one.
Application
5-Amino-3-methyl-1-phenylpyrazole may be used to synthesize:
- substituted pyrazoles
- pyrazolopyridine derivatives
- pyrazolo[3,4,-b]pyridines
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An unexpected chemical behavior of 5-N-(benzotriazol-1-ylmethyl) amino-3-tert-butyl-1-phenylpyrazole.
Abonia R, et al.
Tetrahedron Letters, 43(22), 5617-5620 (2002)
Synthesis of Newly Substituted Pyrazoles and Substituted Pyrazolo [3, 4-b] Pyridines Based on 5-Amino-3-Methyl-1-Phenylpyrazole.
El-Emary TI.
J. Chin. Chem. Soc., 54(2), 507-518 (2007)
Transformation of 4-oxo-4H-[1]-benzopyran-3-carboxaldehydes into pyrazolo [3, 4-B] pyridines.
Stankovicova H, et al.
Journal of Heterocyclic Chemistry, 43(4), 843-848 (2006)
The synthesis of fused and pendant pyrazole heterocyclic compounds from 5-amino-3-methyl-1-phenylpyrazole and their evaluation as fluorescent brightening agents.
Tagdiwala PV and Rangnekar DW.
Journal of Chemical Technology and Biotechnology, 38(2), 77-84 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service