All Photos(2)
About This Item
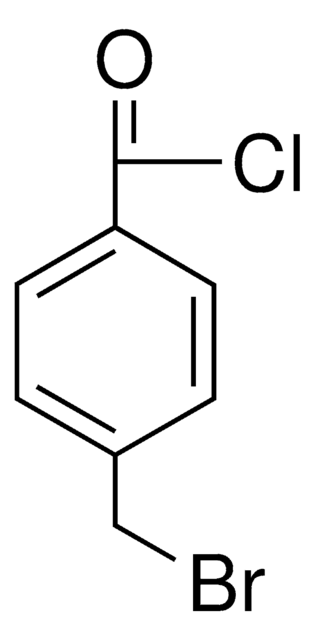
Linear Formula:
BrCH2C6H4C(O)Br
CAS Number:
Molecular Weight:
277.94
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
96%
bp
100-112 °C/0.5 mmHg (lit.)
mp
57-61 °C (lit.)
SMILES string
BrCc1ccc(cc1)C(Br)=O
InChI
1S/C8H6Br2O/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5H2
InChI key
VUYGJYAPDGKPBF-UHFFFAOYSA-N
Related Categories
General description
4-Bromomethyl benzoyl bromide is a para-substituted benzoyl bromide.
Application
4-Bromomethyl benzoyl bromide may be used in the preparation of 4-(bromomethyl)-N-[(S)-3-ethyl-3-hydroxy-1-phenylpentan-2-yl]benzamide and α-methoxy-ω-4-(bromomethyl) benzoic acid ester-poly(ethylene glycol) 2000.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1A
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Systematic MALDI-TOF CID investigation on different substituted mPEG 2000.
Knop K, et al.
Macromolecular Chemistry and Physics, 211(6), 677-684 (2010)
Enantioselective addition of phenylacetylene to aldehydes catalyzed by silica-immobilized titanium (IV) complex of ?-hydroxyamide.
Huang LN, et al.
J. Mol. Catal. A: Chem., 275(1), 9-13 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service