All Photos(2)
About This Item
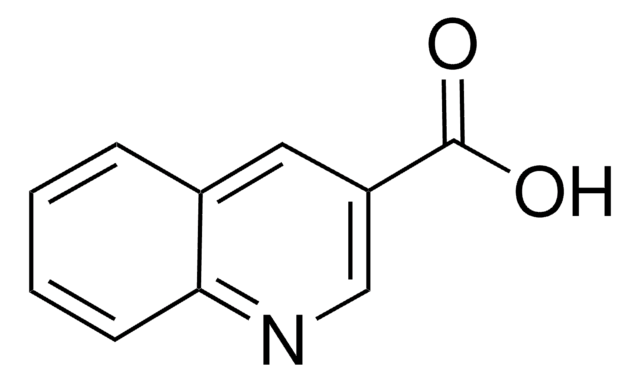
Empirical Formula (Hill Notation):
C10H6ClNO
CAS Number:
Molecular Weight:
191.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
96-98 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
ClC(=O)c1ccc2ccccc2n1
InChI
1S/C10H6ClNO/c11-10(13)9-6-5-7-3-1-2-4-8(7)12-9/h1-6H
InChI key
WFVMVMAUXYOQSW-UHFFFAOYSA-N
Related Categories
General description
Quinaldoyl chloride is a quinaldine derivative.
Application
Quinaldoyl chloride may be used to synthesize 5-chloro-2-(2-quinolinecarboxy)acetophenone and benzoin quinaldate.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of heterocyclic-substituted chromones and related compounds as potential anticancer agents.
D Donnelly et al.
Journal of medicinal chemistry, 8(6), 872-875 (1965-11-01)
Mechanism of the Acid Catalyzed Formation of Aldehydes from Reissert Compounds.
McEwen WE and Hazlett RN.
Journal of the American Chemical Society, 71(6), 1949-1952 (1949)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service