All Photos(1)
About This Item
Linear Formula:
C7H10(=O)2
CAS Number:
Molecular Weight:
126.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.483 (lit.)
bp
254 °C (lit.)
density
1.1 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCCC(=O)C1
InChI
1S/C7H10O2/c8-6-3-1-2-4-7(9)5-6/h1-5H2
InChI key
DBOVMTXPZWVYAQ-UHFFFAOYSA-N
Related Categories
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael addition of 1, 3-cyclopentanedione, 1, 3-cyclohexanedione and 1, 3-cycloheptanedione to 1-(X-phenyl)-2-nitroethylenes.
Hrnciar P and Culak I.
Collection of Czechoslovak Chemical Communications, 49(6), 1421-1431 (1984)
Michael additions of 1, 3-cycloalkanediones to dimethyl acety-lenedicarboxylate.
Hrnciar P, et al.
Chemical Papers, 43(1), 87-95 (1989)
Methylation of 1, 3-cyclopentanedione, 1, 3-cyclohexanedione, and 1, 3-cycloheptanedione with iodomethane in aprotic solvents in the absence and in the presence of 18-crown-6.
Sraga J and Hrnciar P.
Chemical Papers, 35(1), 119-126 (1981)
Allen Y Hong et al.
Tetrahedron, 67(52), 10234-10248 (2012-02-22)
General catalytic asymmetric routes toward cyclopentanoid and cycloheptanoid core structures embedded in numerous natural products have been developed. The central stereoselective transformation in our divergent strategies is the enantioselective decarboxylative alkylation of seven-membered β-ketoesters to form α-quaternary vinylogous esters. Recognition
Evan A Sims et al.
Tetrahedron letters, 52(16), 1871-1873 (2011-06-29)
We report the large-scale synthesis of 1,3-cyclooctanedione in five steps with 29% yield. This molecule is a synthetic precurser to difluorinated cyclooctyne, which participates in a bioorthogonal copper-free click reaction with azides. The final step demonstrates the first successful application
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service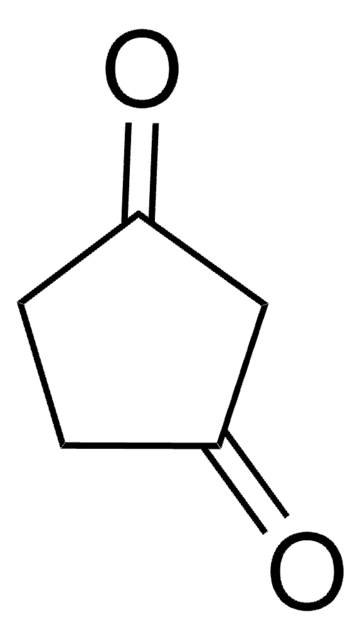




![(Acetonitrile)[(2-biphenyl)di-tert-butylphosphine]gold(I) hexafluoroantimonate](/deepweb/assets/sigmaaldrich/product/structures/216/222/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9/640/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9.png)

