510459
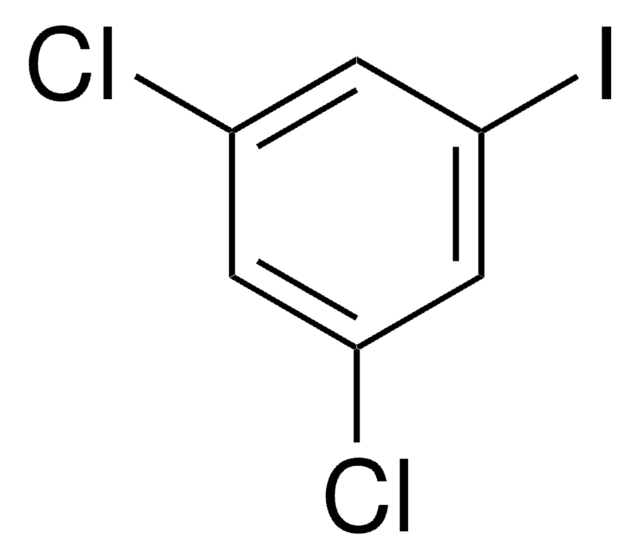
2,6-Dichloroiodobenzene
98%
Synonym(s):
1,3-Dichloro-2-iodobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2C6H3I
CAS Number:
Molecular Weight:
272.90
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
98%
mp
64-67 °C (lit.)
SMILES string
Clc1cccc(Cl)c1I
InChI
1S/C6H3Cl2I/c7-4-2-1-3-5(8)6(4)9/h1-3H
InChI key
ZMPGXSFTXBOKFM-UHFFFAOYSA-N
General description
2,6-Dichloroiodobenzene, also known as 1,3-dichloro-2-iodobenzene, is a halo-substituted benzene. It couples with 1,1′-diaminoferrocene in the presence of a palladium catalyst to form substituted diamide ligands (Fc(NHAr)2).
Application
2,6-Dichloroiodobenzene may be used in the preparation of 2,6-dichlorobenzo[14C]nitrile and 2,6-terphenyl carboxylic acid ligands.
It may be used as a starting material in the multi-step synthesis of:
It may be used as a starting material in the multi-step synthesis of:
- cupped oxacyclophanes
- cuppedophanes
- cappedophanes
- m-terphenyls
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of cuppedophanes and cappedophanes. Two new classes of cyclophanes with molecular cavities.
Vinod T and Hart H.
The Journal of Organic Chemistry, 55(3), 881-890 (1990)
Zirconium complexes incorporating diaryldiamidoferrocene ligands: generation of cationic derivatives and polymerization activity towards ethylene and 1-hexene.
Shafir A and Arnold J.
Inorgorganica Chimica Acta, 345, 216-220 (2003)
Non-interconvertible conformers of cupped oxacyclophanes.
Grewal RS and Hart H.
Tetrahedron Letters, 31(30), 4271-4274 (1990)
Encapsulation of the uranyl dication.
Beer S, et al.
Chemical Science, 1(1), 43-47 (2010)
Oxacyclophanes based on a m-terphenyl framework.
Grewal RS, et al.
The Journal of Organic Chemistry, 57(9), 2721-2726 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service