All Photos(1)
About This Item
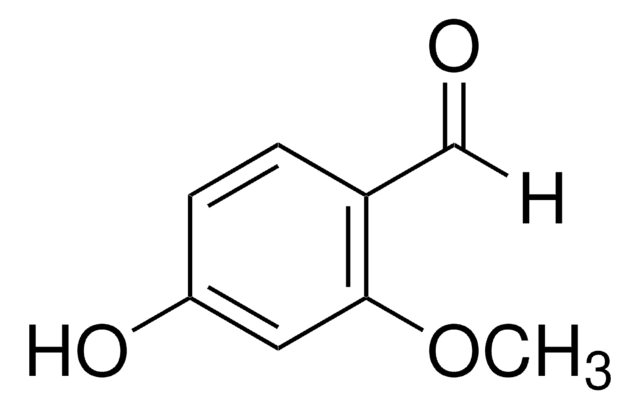
Linear Formula:
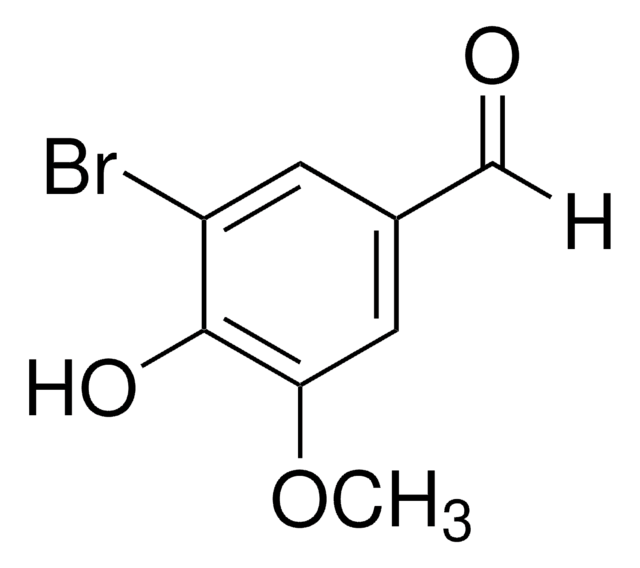
BrC6H2(OH)(OCH3)CHO
CAS Number:
Molecular Weight:
231.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
202-207 °C (lit.)
SMILES string
COc1ccc(C=O)c(Br)c1O
InChI
1S/C8H7BrO3/c1-12-6-3-2-5(4-10)7(9)8(6)11/h2-4,11H,1H3
InChI key
QPDFBPIHEDAUKK-UHFFFAOYSA-N
Related Categories
General description
2-Bromo-3-hydroxy-4-methoxybenzaldehyde, also known as 2-bromo-isovanillin, can be synthesized by the bromination of 3-hydroxy-4-methoxybenzaldehyde.
application
2-Bromo-3-hydroxy-4-methoxybenzaldehyde (2-Bromo-isovanillin) may be used in the preparation of:
- 2-hydroxy-3-methoxybenzaldehyde semicarbazone (HMBS)
- 2-cyclopentyl-7-methoxy-1-benzofuran-4-carbaldehyde
- 3-(benzyloxy)-2-bromo-4-methoxybenzaldehyde
- pareitropone
- denbinobin
- (±)-codeine
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Total synthesis of pareitropone via radical anion coupling.
Hong SK, et al.
Organic Letters, 12(17), 3954-3956 (2010)
Growth, spectral, and thermal characterization of 2-hydroxy-3-methoxybenzaldehyde semicarbazone.
Binil PS, et al.
Journal of Thermal Analysis and Calorimetry, 112(2), 913-919 (2013)
A concise synthesis of denbinobin.
Wang YC, et al.
Tetrahedron Letters, 46(47), 8103-8104 (2005)
Synthesis and preliminary biological evaluation of novel taspine derivatives as anticancer agents.
Zhang J, et al.
European Journal of Medicinal Chemistry, 45(7), 2798-2805 (2010)
Concise syntheses of (-)-galanthamine and (?)-codeine via intramolecular alkylation of a phenol derivative.
Magnus P, et al.
Journal of the American Chemical Society, 131(44), 16045-16047 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service