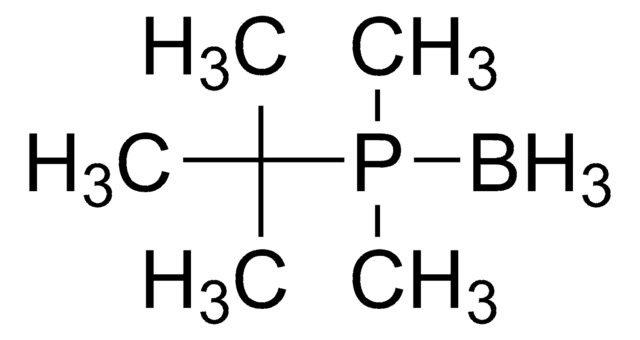
449563
Borane diphenylphosphine complex
98%
Synonym(s):
(Diphenylphosphine)trihydroboron
About This Item
Recommended Products
assay
98%
form
solid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Reductions
reagent type: reductant
bp
148-150 °C/10 mmHg (lit.)
mp
47-50 °C (lit.)
SMILES string
B.[H]P(c1ccccc1)c2ccccc2
InChI
1S/C12H11P.BH3/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12;/h1-10,13H;1H3
InChI key
XUFGOLYVDOBYLK-UHFFFAOYSA-N
Application
- Nucleophilic addition reactions (anion)
- Catalytic dehydrogenation in the presence of ruthenium bidentate phosphine compelxes
- Deprotonation reactions
- Catalytic coupling with alkynyl bromides
- Catalyst-free Staudinger ligation for indirect 18F-radiolabeling
- Preparation of chiral phosphine-phosphite bidentate ligands with biphenyl backbone
- Reactions with frustrated lewis pair combinations of group 14 triflates and sterically hindered nitrogen bases
signalword
Danger
hcodes
pcodes
Hazard Classifications
Water-react 2
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service