328014
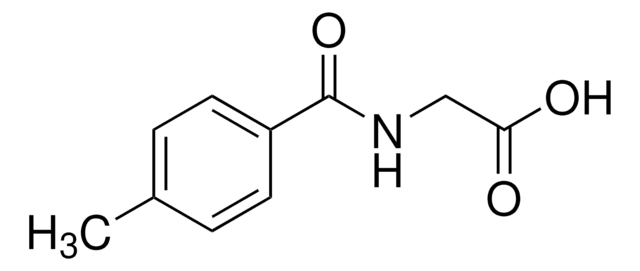
3-Methylhippuric acid
98%
Synonym(s):
N-(3-Methylbenzoyl)glycine, m-Toluric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4CONHCH2CO2H
CAS Number:
Molecular Weight:
193.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
138-140 °C (lit.)
SMILES string
Cc1cccc(c1)C(=O)NCC(O)=O
InChI
1S/C10H11NO3/c1-7-3-2-4-8(5-7)10(14)11-6-9(12)13/h2-5H,6H2,1H3,(H,11,14)(H,12,13)
InChI key
YKAKNMHEIJUKEX-UHFFFAOYSA-N
Related Categories
General description
3-Methylhippuric acid is also referred as m-methyl-hippuric acid. It is major product of xylene biotransformation in urine.
Application
3-Methylhippuric acid was employed as biological marker in studies on occupational exposure to xylene (solvent).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D de Carvalho et al.
International archives of occupational and environmental health, 63(1), 33-37 (1991-01-01)
The industrial solvents, toluene and xylene, have physicochemical properties that can be hazardous to the workers exposed. Since hippuric acid and m-methyl-hippuric acid represent the products of toluene and xylene biotransformation in urine, they are used as biological markers in
R Tardif et al.
Occupational and environmental medicine, 51(3), 187-191 (1994-03-01)
This study was undertaken to determine whether previous subacute treatment with ethanol could modify the kinetics of m-xylene in humans. A group of six volunteers was exposed twice to either 100 or 400 ppm of m-xylene during two hours (between
Determination of methylhippuric acid in human urine by high-performance liquid chromatography and by isotachophoresis.
J Sollenberg et al.
Journal of chromatography, 343(2), 419-423 (1985-10-11)
Possible preferential metabolism of xylene isomers following occupational exposure to mixed xylenes.
M J Miller et al.
International archives of occupational and environmental health, 72(2), 89-97 (1999-04-10)
Solvent exposures commonly involve mixtures of substances or mixtures of isomers of a single solvent. These may be metabolised through common pathways, resulting in the potential for metabolic interactions. These may then lead to accumulation of solvent or metabolic intermediates
A Astier
Journal of chromatography, 573(2), 318-322 (1992-01-17)
A high-performance liquid chromatographic method is described for the simultaneous determination of six urinary metabolites of several aromatic chemicals: phenol (from benzene), hippuric acid (from toluene), 3-methylhippuric acid (from xylene), mandelic and phenylglyoxylic acid (from styrene) and 4-nitrophenol (from nitrobenzene).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service