300756
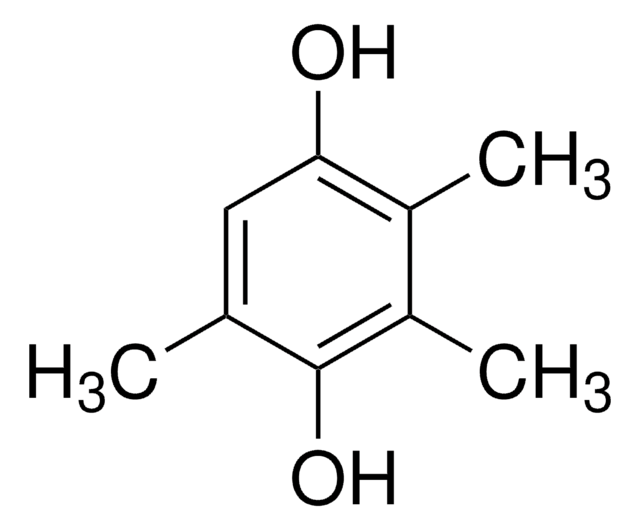
2,3-Dimethylhydroquinone
97%
Synonym(s):
1,4-Dihydroxy-2,3-dimethylbenzene, 2,3-Dimethyl-1,4-benzenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C6H2-1,4-(OH)2
CAS Number:
Molecular Weight:
138.16
Beilstein/REAXYS Number:
636976
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
97%
form
powder
mp
223-225 °C (lit.)
SMILES string
Cc1c(C)c(O)ccc1O
InChI
1S/C8H10O2/c1-5-6(2)8(10)4-3-7(5)9/h3-4,9-10H,1-2H3
InChI key
BXJGUBZTZWCMEX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dimethylhydroquinone is an alkyl p-hydroquinone that can be used as a chain breaking antioxidant and an electron donor for redox intermediates. It acts as an antioxidant due to its characteristic to terminate kinetic chains on reaction with peroxy radicals.
Application
2,3-Dimethylhydroquinone can be used as an antioxidant for lipid peroxidation. It is also used in the synthesis of benzofuran-5-ols which can further be utilized as antifungal agents in biological applications.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and antifungal activity of benzofuran-5-ols
Ryu C, et al.
Bioorganic & Medicinal Chemistry Letters, 20(22), 6777-6780 (2010)
Substituted p-hydroquinones as inhibitors of lipid peroxidation
Roginsky V, et al.
Chemistry and Physics of Lipids, 125(1), 49-58 (2003)
Electrochemical oxidation of 2, 3-dimethylhydroquinone in the presence of 1, 3-dicarbonyl compounds
Hosseiny D, et al.
The Journal of Organic Chemistry, 71(5), 2139-2142 (2006)
Alnald Javier et al.
Dalton transactions (Cambridge, England : 2003), 43(39), 14798-14805 (2014-08-28)
Previous studies, based on thin-layer electrochemistry (TLE), in situ scanning tunneling microscopy (EC-STM), high-resolution electron energy loss spectroscopy (HREELS) and density functional theory (DFT) computations, on the chemical adsorption of hydroquinone from aqueous solutions onto atomically smooth Pd (and Pt)
U Burner et al.
Redox report : communications in free radical research, 5(4), 185-190 (2000-09-20)
Myeloperoxidase is very susceptible to reducing radicals because the reduction potential of the ferric/ferrous redox couple is much higher compared with other peroxidases. Semiquinone radicals are known to reduce heme proteins. Therefore, the kinetics and spectra of the reactions of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service