All Photos(1)
About This Item
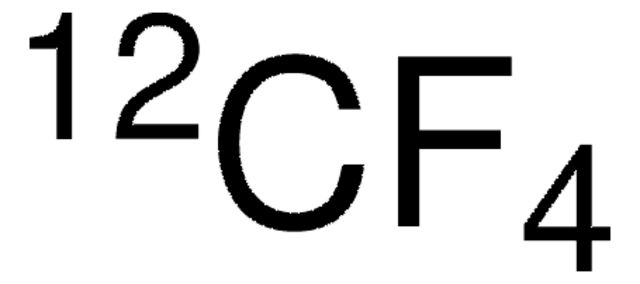
Linear Formula:
CF4
CAS Number:
Molecular Weight:
88.00
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.04 (vs air)
assay
99.9%
bp
−130 °C (lit.)
mp
−184 °C (lit.)
SMILES string
FC(F)(F)F
InChI
1S/CF4/c2-1(3,4)5
InChI key
TXEYQDLBPFQVAA-UHFFFAOYSA-N
General description
Carbon tetrafluoride (CF4), also known as tetrafluoromethane, belongs to the class of fluoromethanes. It can be prepared by the reaction of silicon carbide with fluorine. It is used as a refrigerant.
Application
Carbon tetrafluoride can be used in fluorine chemistry for the preparation of organofluorine compounds. It is also used as a solvent in low-temperature reactions . During the manufacture of electronics devices, CF4 plasma is widely used to etch a variety of materials.
Packaging
Supplied in a carbon steel lecture bottle with a CGA180M/CGA110F needle valve installed.
Compatible with the following:
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
Legal Information
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
also commonly purchased with this product
Product No.
Description
Pricing
control valve
hose barb
Product No.
Description
Pricing
purge valve
Product No.
Description
Pricing
recommended
Product No.
Description
Pricing
signalword
Warning
hcodes
pcodes
Hazard Classifications
Press. Gas Liquefied gas
Storage Class
2A - Gases
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D S Rawat et al.
Langmuir : the ACS journal of surfaces and colloids, 24(23), 13465-13469 (2008-10-29)
We present results for the isothermal adsorption kinetics of methane, hydrogen, and tetrafluoromethane on closed-ended single-walled carbon nanotubes. In these experiments, we monitor the pressure decrease as a function of time as equilibrium is approached, after a dose of gas
Erich A Müller
Environmental science & technology, 39(22), 8736-8741 (2005-12-06)
Grand canonical Monte Carlo simulations are reported for low molecular weight perfluorocarbons (C2F6 and CF4) and their dilute (10% molar) mixtures in N2 adsorbing unto slitlike graphite pores. Adsorption isotherms and selectivity plots are shown for various temperatures, pressures, and
Fluorination of calcium cyanamide: a convenient laboratory scale synthesis of carbon tetrafluoride
Tornieporth-Oetting IC, et al.
Inorganic Chemistry, 31(18), 3864-3864 (1992)
A Bishop et al.
Nuclear medicine and biology, 23(3), 189-199 (1996-04-01)
The production of 18F electrophilic reagents via the 18O(p,n)18F reaction has been investigated in small-volume target bodies made of aluminum, copper, gold-plated copper and nickel, having straight or conical bore shapes. Three irradiation protocols-single-step, two-step and modified two-step-were used for
R Eloy et al.
Journal of cataract and refractive surgery, 19(3), 364-370 (1993-05-01)
The inflammatory cell response of tetrafluorocarbon (CF4) plasma surface modification of poly(methyl methacrylate) intraocular lenses (IOLs) was investigated in vitro. After two hours of lens contact with human granulocytes, scanning electron microscopy showed significantly less cell activation and granulocyte adhesion
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service