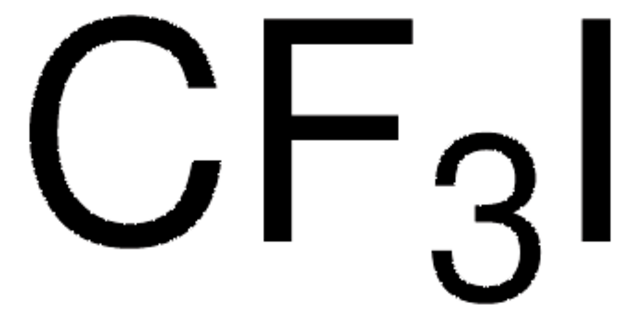295337
Trifluoromethane
≥98%
Synonym(s):
Fluoroform, HFC-23
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CHF3
CAS Number:
Molecular Weight:
70.01
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.43 (vs air)
vapor pressure
635 psi ( 21 °C)
assay
≥98%
bp
−84 °C (lit.)
mp
−160 °C (lit.)
SMILES string
FC(F)F
InChI
1S/CHF3/c2-1(3)4/h1H
InChI key
XPDWGBQVDMORPB-UHFFFAOYSA-N
Packaging
Supplied in a carbon steel lecture bottle with a CGA180M/CGA110F needle valve installed.
Compatible with the following:
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
Legal Information
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
also commonly purchased with this product
Product No.
Description
Pricing
control valve
hose barb
Product No.
Description
Pricing
purge valve
Product No.
Description
Pricing
recommended
Product No.
Description
Pricing
signalword
Warning
hcodes
pcodes
Hazard Classifications
Press. Gas Liquefied gas
Storage Class
2A - Gases
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mark A Honey et al.
The Journal of organic chemistry, 77(3), 1396-1405 (2012-01-24)
Ethyl 2-diazo-4,4,4-trifluoro-3-oxobutanoate is a highly versatile intermediate for the synthesis of a wide range of trifluoromethyl heterocycles. With the use of rhodium(II) or copper(II) catalyzed carbene X-H insertion reactions as key steps, a diverse set of trifluoromethyl-oxazoles, -thiazoles, -imidazoles, -1,2,4-triazines
Shun Noritake et al.
Organic & biomolecular chemistry, 7(17), 3599-3604 (2009-08-14)
Chiral nonracemic guanidines act as Brønsted bases to generate guanidinium enolates for the enantioselective electrophilic trifluoromethylation of beta-keto esters by means of S-(trifluoromethyl)dibenzothiophenium tetrafluoroborate (Umemoto reagent) with good enantioselectivity of 60-70% range. Despite the fact that the ees are still
Thierry Milcent et al.
Organic & biomolecular chemistry, 8(13), 3025-3030 (2010-05-12)
Trifluoromethyl nitrones were obtained in high yields by condensation of various hydroxylamines with trifluoroacetaldehyde hydrate. The nucleophilic diastereoselective additions of organometallic reagents to these nitrones afforded the corresponding optically active trifluoroethyl hydroxylamines in good yields.
Yongwei Wu et al.
Journal of the American Chemical Society, 134(35), 14334-14337 (2012-08-22)
A new approach toward the asymmetric synthesis of optically active trifluoromethylated amines was enabled by an unprecedented, highly enantioselective catalytic isomerization of trifluoromethyl imines with a new chiral organic catalyst. Not only aryl but also alkyl trifluoromethylated amines could be
Charles S Thomoson et al.
The Journal of organic chemistry, 78(17), 8904-8908 (2013-08-13)
Fluoroform, CHF3, a non-ozone-depleting, nontoxic, and inexpensive gas can be used as a difluorocarbene source in a process for the conversion of phenols and thiophenols to their difluoromethoxy and difluorothiomethoxy derivatives. The reactions are carried out at moderate temperatures and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service