All Photos(2)
About This Item
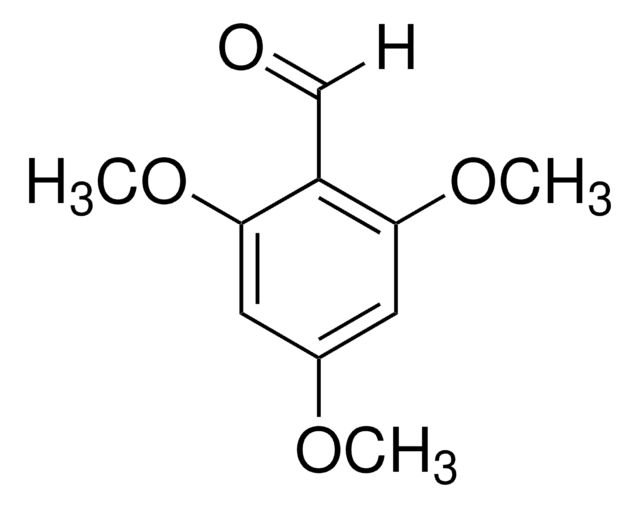
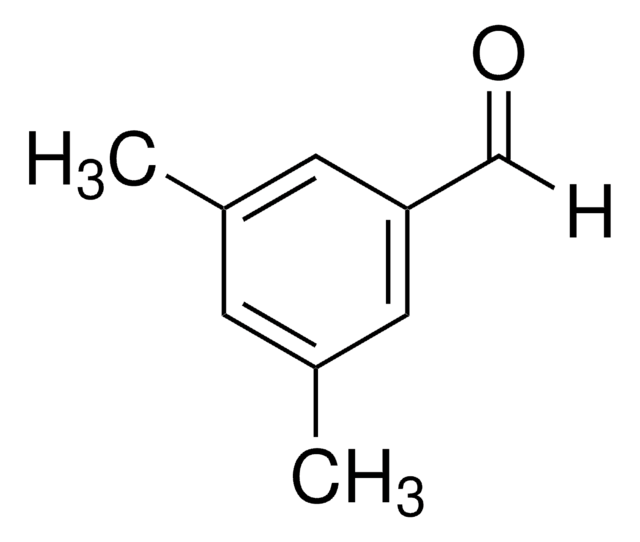
Linear Formula:
(CH3O)2C6H3CHO
CAS Number:
Molecular Weight:
166.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
bp
285 °C (lit.)
mp
96-98 °C (lit.)
SMILES string
COc1cccc(OC)c1C=O
InChI
1S/C9H10O3/c1-11-8-4-3-5-9(12-2)7(8)6-10/h3-6H,1-2H3
Inchi Key
WXSGQHKHUYTJNB-UHFFFAOYSA-N
General description
Demethylation of 2,6-dimethoxybenzaldehydes with magnesium iodide etherate has been studied.
Application
2,6-Dimethoxybenzaldehyde has been used in the preparation of 2,6-dihydroxybenzaldehyde by demethylation with AlBr3.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Regioselective demethylation of 2, 6-dimethoxybenzaldehydes with magnesium iodide etherate.
Yamaguchi S, et al.
Tetrahedron Letters, 40(41), 7363-7365 (1999)
Synthesis of facially-encumbered porphyrins. An approach to light-harvesting antenna complexes.
Wagner RW, et al.
Tetrahedron Letters, 32(14), 1703-1706 (1991)
Olga V Kupriyanova et al.
Drug testing and analysis, 12(8), 1154-1170 (2020-05-18)
N-(2-Methoxybenzyl)-2,5-dimethoxyphenethylamines (NBOMes) are synthetic phenethylamine derivatives emerging on the global drug market and reported to be associated with untoward effects in people who use drugs. Its action involves agonism at serotonin 5-HT2A receptors, affecting cognitive and behavioral processes. However, certain
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service