All Photos(1)
About This Item
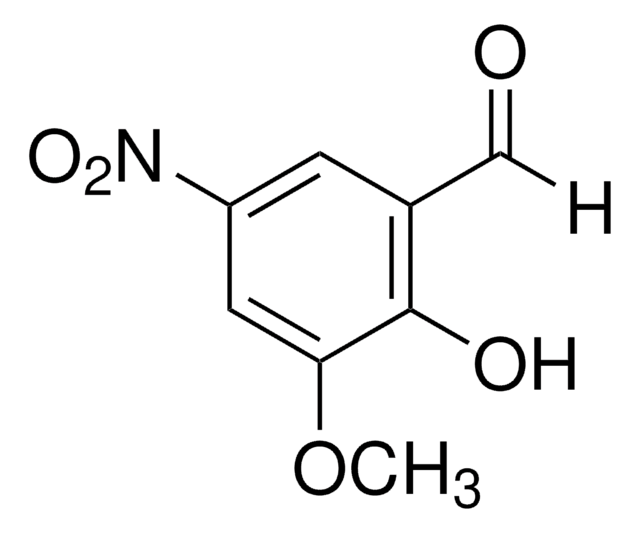
Linear Formula:
(O2N)2C6H2-2-(OH)CHO
CAS Number:
Molecular Weight:
212.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
68-70 °C (lit.)
SMILES string
Oc1c(C=O)cc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H4N2O6/c10-3-4-1-5(8(12)13)2-6(7(4)11)9(14)15/h1-3,11H
InChI key
FLJXIBHYDIMYRS-UHFFFAOYSA-N
Application
3,5-Dinitrosalicylaldehyde has been used in the preparation of:
- salicyldimine ligand via Schiff base condensation with allyl-substituted aniline
- 3-hydroxycoumarins
- chromogenic proteinase substrates
- Ruthenium(II) chiral Schiff base complexes
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N G Gallegos et al.
Journal of biochemical and biophysical methods, 33(1), 31-41 (1996-10-15)
To search for new proteinases in Bacillus subtilis we have developed a general method for synthesizing chromogenic proteinase substrates using 3,5-dinitrosalicylaldehyde (DNSA). Hammersten casein and soluble protein from extracts from B. subtilis cells were labeled with DNSA in the presence
Dao Zhang et al.
Chemical communications (Cambridge, England), (6)(6), 574-575 (2002-07-18)
A new family of self-immobilized ethylene polymerization catalysts, derived from neutral, single-component salicylaldiminato phenyl nickel complexes, is described.
Synthesis, physico-chemical studies and solvent-dependent enantioselective epoxidation of 1, 2-dihydronaphthalene catalysed by chiral Ruthenium (II) Schiff base complexes
Kureshy RI, et al.
J. Mol. Catal. A: Chem., 150(1), 163-173 (1999)
Notes-3-Hydroxycoumarins.
Trivedi K and Sethan S.
The Journal of Organic Chemistry, 25(10), 1817-1819 (1960)
Ryan J DiRisio et al.
Dalton transactions (Cambridge, England : 2003), 46(31), 10418-10425 (2017-07-27)
Two cobalt(iii) complexes containing inexpensive Schiff-base ligands have been found to be active for proton reduction at low overpotentials. The dinitro and tetranitro derivatized Schiff-base complexes show catalytic activity at -0.96 V and -1.1 V vs. Fc
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service