244988
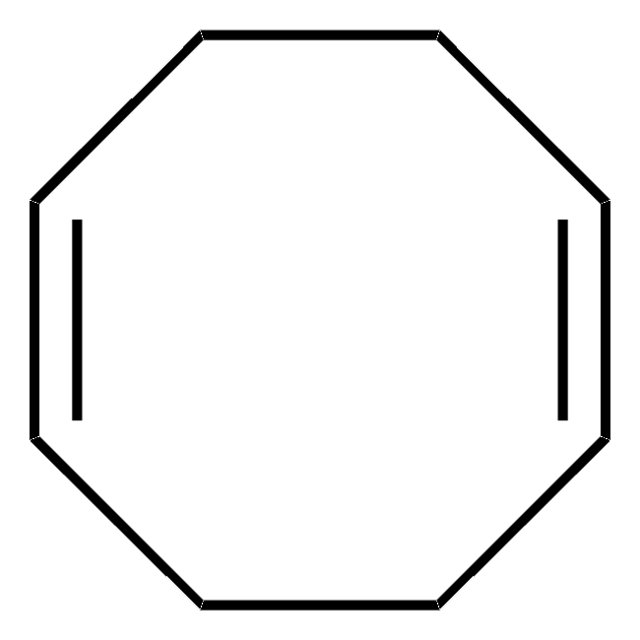
Bis(1,5-cyclooctadiene)nickel(0)
Synonym(s):
Bis(cyclooctadiene)nickel, Ni(COD)2
About This Item
Recommended Products
Quality Level
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
parameter
temperature sensitive
mp
60 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
[Ni].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.Ni/c2*1-2-4-6-8-7-5-3-1;/h2*1-2,7-8H,3-6H2;/b2*2-1-,8-7-;
InChI key
JRTIUDXYIUKIIE-KZUMESAESA-N
Application
- Oxidative addition reactions
Catalyst for:
- Asymmetric α-arylation and heteroarylation of ketones with chloroarenes
- Cross-coupling reactions
- Regioselective and stereoselective carboxylation/cyclization of allenyl aldehydes under a carbon dioxide atmosphere
- Methyl carboxylation of homopropargylic alcohols
- Stereoselective borylative ketone-diene coupling
- Cycloaddition of benzamides with internal alkynes
related product
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Flam. Sol. 1 - Skin Sens. 1 - STOT RE 1
target_organs
Lungs
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Effective Csp2- and Csp-hybridized coupling reactions established for catalysis, expanding to multi-step Csp3-coupling.
Nickel complexes catalyze various synthetic reactions like oxidative addition, C-H activation, and cross-coupling.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service