All Photos(1)
About This Item
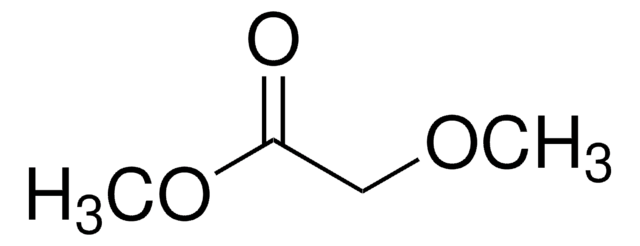
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
Beilstein/REAXYS Number:
103177
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
99%
form
liquid
refractive index
n20/D 1.409 (lit.)
bp
129-130 °C (lit.)
density
1.092 g/mL at 25 °C (lit.)
SMILES string
COC1OCCO1
InChI
1S/C4H8O3/c1-5-4-6-2-3-7-4/h4H,2-3H2,1H3
Inchi Key
VRAYTNFBRROPJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
application
2-Methoxy-1,3-dioxolane was used in the synthesis of :
- [4,5-bis(hydroxymethyl)-1,3-dioxolan-2-yl]nucleosides, potential inhibitors of HIV
- diastereoisomeric cyclic acetals
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of highly substituted 2, 6-anti-configured tetrahydropyrans. First steps towards an efficient access to amphidinol 3 ring system.
Dubost C, et al.
Tetrahedron Letters, 46(23), 4005-4009 (2005)
Jonas Brånalt et al.
The Journal of organic chemistry, 61(11), 3599-3603 (1996-05-31)
The synthesis of 1,3-dioxolan-2-ylnucleosides and related chemistry is described. We have shown that 2-methoxy-1,3-dioxolane (6) reacts with silylated thymine and trimethylsilyl triflate to give the acyclic formate ester 1-[2-(formyloxy)ethyl]thymine (8) rather than 1-(1,3-dioxolan-2-yl)thymine (7). A tentative mechanism which could explain
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service