The theoretical content of Ru in Ruthenium(III) chloride (RuCl3) should be 101.07 / 207.43 = ~48.72%, which falls into the specification range of this product. The other half of this product is mainly Cl ions. The Ru content is tested on a lot-to-lot basis. The purity can be roughly calculated using Ru content divided by 48.72%.
208523
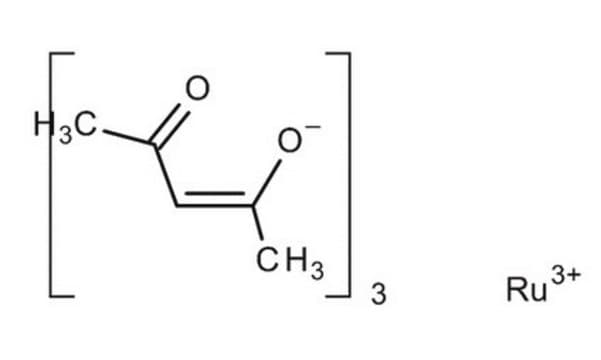
Ruthenium(III) chloride
Ru content 45-55%
Synonym(s):
Ruthenium trichloride
Select a Size
About This Item
Recommended Products
Quality Level
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Atom Transfer Radical Polymerization (ATRP)
density
3.11 g/mL at 25 °C (lit.)
SMILES string
Cl[Ru](Cl)Cl
InChI
1S/3ClH.Ru/h3*1H;/q;;;+3/p-3
InChI key
YBCAZPLXEGKKFM-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization.
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
-
I have purchasedRuthenium(III) chloride, Ru content 45-55% from you company. However, I am confused on the statement of "Ru content 45-55%". If Ru is only hald of the materials, what are the other half? What is the purity of the chemical?
1 answer-
Helpful?
-
-
What is the purity of this chemical?
1 answer-
The Ruthenium content for this product ranges from 45-55 %. This value is determined by gravimetric analysis and ICP. The exact amount is lot-specific and is reported on the Certificate of Analysis of the batch received. The overall purity can be calculated by using the molecular weight of the Ruthenium(III) chloride.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service