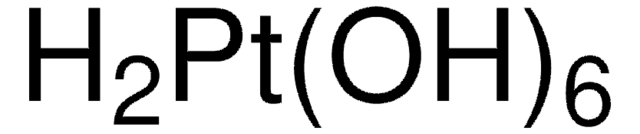Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
206032
Platinum(IV) oxide
Synonym(s):
Adam’s catalyst, NSC 402624, Platinic oxide, Platinum dioxide
Select a Size
About This Item
Recommended Products
form
solid
Quality Level
composition
Pt, 81-83%
reaction suitability
core: platinum
reagent type: catalyst
greener alternative product characteristics
Designing Safer Chemicals
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
surface area
≥60 m2/g
mp
450 °C (lit.)
greener alternative category
, Aligned
SMILES string
O=[Pt]=O
InChI
1S/2O.Pt
InChI key
YKIOKAURTKXMSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Hydrohydrazination reactions
- Oxidation of sugars
- Low-temperature oxidation of carbon monoxide
- Oxygen reduction reaction
- Hydrogenation reactions
- Hydrogenolysis of the cyclopropyl group into an isopropyl group
- Hydroxycyclopropanation reactions
signalword
Danger
hcodes
Hazard Classifications
Ox. Sol. 1
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
Is Product 206032, Platinum (IV) oxide, water soluble?
1 answer-
It is insoluble in water.
Helpful?
-
-
Are there any safety concerns with Product 206032, Platinum (IV) oxide?
1 answer-
After exposure to hydrogen, the resulting platinum black can be pyrophoric. Therefore, it should not be allowed to dry and all exposure to oxygen should be minimized.
Helpful?
-
-
How can Product 206032, Platinum (IV) oxide, be used as a catalyst?
1 answer-
Platinum(IV) oxide can be used as a catalyst in hydrogenations. The actual catalyst is platinum black which is formed in situ by reduction of the PtO2 by the hydrogen used for the hydrogenation. It is especially useful for reduction at room temperature and hydrogen pressures up to 4 atmospheres. It is suitable for the reduction of double and triple bonds, aromatic nuclei, carbonyl groups, nitro groups, and nitriles.
Helpful?
-
-
In which solvents is Product 206032, Platinum (IV) oxide, soluble?
1 answer-
Per the the chemicals encyclopedia published by the Royal Society of Chemistry, 13th Edition, when freshly precipitated, Platinum(IV) Oxide is soluble in concentrated acids and dilute phosphoric acid, especially when warmed. It is easily soluble in dilute solutions of potassium hydroxide.
Helpful?
-
-
Can the Platinum be recovered from Product 206032, Platinum (IV) oxide?
1 answer-
Internet sources do indicate that Platinum can be recovered from spent catalyst by conversion to ammonium chloroplatinate using aqua regia followed by ammonia. However, we do have a process for doing so.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service