All Photos(1)
About This Item
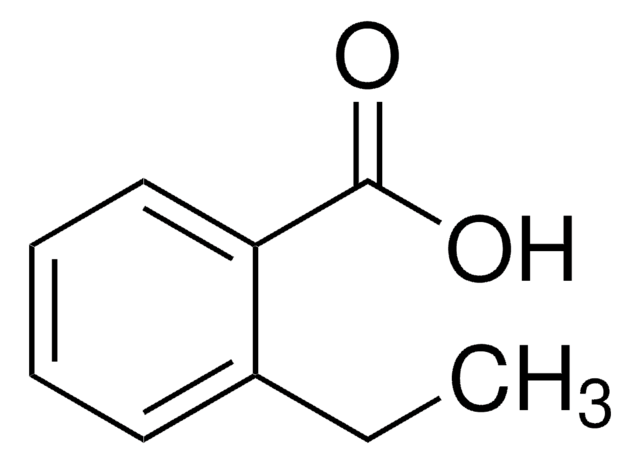
Linear Formula:
C2H5C6H4CO2H
CAS Number:
Molecular Weight:
150.17
Beilstein/REAXYS Number:
2041840
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
mp
112-113 °C (lit.)
SMILES string
CCc1ccc(cc1)C(O)=O
InChI
1S/C9H10O2/c1-2-7-3-5-8(6-4-7)9(10)11/h3-6H,2H2,1H3,(H,10,11)
InChI key
ZQVKTHRQIXSMGY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Ethylbenzoic acid reacts with lanthanum nitrate in aqueous solution to yield the polymer catena-poly[[aqua(4-ethylbenzoic acid-κO)lanthanum(III)]-tri-μ-4-ethylbenzoato].
Application
4-Ethylbenzoic acid was used in the synthesis of ethyl 4-vinyl-α-cyano-β-phenylcinnamate. It was also used to functionalize the edge of “pristine” graphite in the presence of polyphosphoric acid/phosphorus pentoxide.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Juan Yang et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 2), m183-m184 (2010-01-01)
The reaction of lanthanum nitrate and 4-ethyl-benzoic acid (EBAH) in aqueous solution yielded the title polymer, [La(C(9)H(9)O(2))(3)(C(9)H(10)O(2))(H(2)O)](n). The asymmetric unit contains one La(III) atom, three 4-ethyl-benzoate (EBA) ligands, one neutral EBAH ligand and one coordinated water mol-ecule. Each La(III) ion
Functional Polymers. VII. Ethyl 4-Vinyl-α-cyano-β-phenylcinnamate.
Sumida Y and Vogl O.
Polymer Journal, 13(6), 521-536 (1981)
José María Moreno et al.
Chemical science, 10(7), 2053-2066 (2019-03-08)
Novel MOF-type materials with different morphologies based on assembled 1D organic-inorganic sub-domains were prepared using specific monodentate benzylcarboxylate spacers with functional substituents in the para-position as structure modulating agents. The combination of electron-withdrawing or electron-donating functions in the organic spacers
J L Ramos et al.
Science (New York, N.Y.), 235(4788), 593-596 (1987-01-30)
Increasing quantities of man-made organic chemicals are released each year into the biosphere. Some of these compounds are both toxic and relatively resistant to physical, chemical, or biological degradation, and they thus constitute an environmental burden of considerable magnitude. Genetic
Ruthenium(II)-catalyzed synthesis of hydroxylated arenes with ester as an effective directing group.
Yiqing Yang et al.
Organic letters, 14(11), 2874-2877 (2012-05-16)
An unprecedented Ru(II) catalyzed ortho-hydroxylation has been developed for the facile synthesis of a variety of multifunctionalized arenes from easily accessible ethyl benzoates with ester as an efficient directing group. Both the TFA/TFAA cosolvent system and oxidants serve as the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service