125504
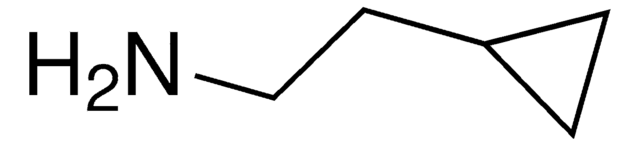
Cyclopropylamine
98%
Synonym(s):
Aminocyclopropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C3H5NH2
CAS Number:
Molecular Weight:
57.09
Beilstein/REAXYS Number:
741858
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
assay:
98%
Recommended Products
vapor pressure
4.67 psi ( 20 °C)
Quality Level
assay
98%
form
liquid
autoignition temp.
527 °F
refractive index
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
SMILES string
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
InChI key
HTJDQJBWANPRPF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclopropylamine(CPA) has been used in the synthesis of N-[4-(4-fluoro)phenyl-2-aminothiazol-5-yl]pyrimidin-2-yl-alkylamine derivatives. It has been used in the synthesis of Pt(CPA)2(bismethylthiomethylenepropanedioate) and Pt(CPA)2(bisethylthiomethylenepropanedioate) complexes.
Biochem/physiol Actions
Cyclopropylamine inactivates cytochrome P450 enzymes by a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. It is a mechanism-based inhibitor of quinoprotein methylamine dehydrogenase from Paracoccus denitrificans.
accessory
Product No.
Description
Pricing
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
33.8 °F - closed cup
flash_point_c
1 °C - closed cup
ppe
Faceshields, Gloves, Goggles
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Coordination Mode vs. Anticancer Activity of the Platinum (II) Complexes Involving Sulfur-Containing Ylidenemalonate Ligands.
Sakai N, et al.
Bull. Korean Chem. Soc., 19(12), 1377-1379 (1998)
Jo-Yanne Le Berre et al.
PloS one, 12(12), e0190341-e0190341 (2017-12-28)
Little is known about the responses of plant roots to filamentous pathogens, particularly to oomycetes. To assess the molecular dialog established between the host and the pathogen during early stages of infection, we investigated the overall changes in gene expression
Christopher L Shaffer et al.
Journal of the American Chemical Society, 124(28), 8268-8274 (2002-07-11)
The role of single electron transfer (SET) in P450-catalyzed N-dealkylation reactions has been studied using the probe substrates N-cyclopropyl-N-methylaniline (2a) and N-(1'-methylcyclopropyl)-N-methylaniline (2b). In earlier work, we showed that SET oxidation of 2a by horseadish peroxidase leads exclusively to products
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service