125415
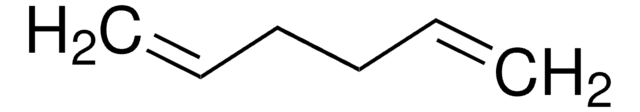
1,4-Cyclohexadiene
97%
Synonym(s):
1,4-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein/REAXYS Number:
1900733
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
assay:
97%
Recommended Products
Quality Level
assay
97%
form
liquid
contains
~0.1% hydroquinone as stabilizer
impurities
3% benzene
refractive index
n20/D 1.472 (lit.)
bp
88-89 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=CCC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-2,5-6H,3-4H2
InChI key
UVJHQYIOXKWHFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,4-Cyclohexadiene is an effective hydrogen donor for catalytic hydrogenation reactions. It can rapidly replace benzyl groups of N-benzyloxycarbamates, benzyl esters, benzyl ethers and benzyl amines with hydrogen. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
application
1,4-Cyclohexadiene (1,4-CHD) was used to study the formation of parent ion from heavy fragmentation of 1,4-CHD on irradiation with a high-intensity laser pulse.
Useful for the reduction of radical intermediates formed in electron-transfer mediated ring-opening reactions
signalword
Danger
hcodes
Hazard Classifications
Carc. 1A - Flam. Liq. 2 - Muta. 1B - STOT RE 2
target_organs
Blood
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
19.4 °F - closed cup
flash_point_c
-7 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A key factor in parent and fragment ion formation on irradiation with an intense femtosecond laser pulse.
Harada H, et al.
Chemical Physics Letters, 342(5), 563-570 (2001)
Organometallics, 25, 5456-5456 (2006)
Kyung-Bin Cho et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(33), 10444-10453 (2012-06-21)
The experimentally measured bimolecular reaction rate constant, k(2), should in principle correlate with the theoretically calculated rate-limiting free energy barrier, ΔG(≠), through the Eyring equation, but it fails quite often to do so due to the inability of current computational
Kazutada Ikeuchi et al.
Organic letters, 14(23), 6016-6019 (2012-11-15)
Asymmetric bromolactonization of prochiral cyclohexadiene derivatives with N-bromosuccimide proceeded in the presence of (DHQD)(2)PHAL as a chiral catalyst to afford the corresponding bromolactones with up to 93% ee. This reaction was also applicable to the kinetic resolution of a racemic
Unravelling the details of vitamin D photosynthesis by non-adiabatic molecular dynamics simulations.
Enrico Tapavicza et al.
Physical chemistry chemical physics : PCCP, 13(47), 20986-20998 (2011-10-25)
We investigate the photodynamics of vitamin D derivatives by a fully analytical implementation of the linear response time-dependent density functional theory surface hopping method (LR-TDDFT-SH). Our study elucidates the dynamics of the processes involved in vitamin D formation at the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)