108146
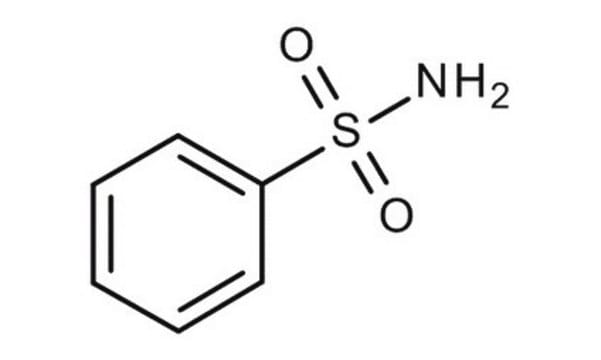
Benzenesulfonamide
≥98%
Synonym(s):
Phenylsulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SO2NH2
CAS Number:
Molecular Weight:
157.19
Beilstein/REAXYS Number:
1100566
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39093209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98%
form
solid
mp
149-152 °C (lit.)
solubility
methanol: soluble 25 mg/mL
SMILES string
NS(=O)(=O)c1ccccc1
InChI
1S/C6H7NO2S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H,(H2,7,8,9)
InChI key
KHBQMWCZKVMBLN-UHFFFAOYSA-N
Gene Information
human ... CA1(759)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Benzenesulfonamide was used to develop analytical method for simultaneous determination of benzotriazole, benzothiazole and benzenesulfonamide contaminants in environmental waters.
Biochem/physiol Actions
Benzenesulfonamide is an inhibitor of human carbonic anhydrase B. Benzenesulfonamide derivatives are effective in the treatment of proliferative diseases such as cancer. It is used in the synthesis of dyes, photochemicals and disinfectants.
Preparation Note
Benzenesulfonamide dissolves in methanol at a concentration of 25 mg/ml to form a clear, colourless solution.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Asha Chandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 92, 84-90 (2012-03-27)
The FT-IR and FT-Raman spectra of (E)-N-carbamimidoyl-4-((4-methoxybenzylidene)amino)benzenesulfonamide were recorded and analyzed. Geometry and harmonic vibrational wavenumbers were calculated theoretically using Gaussian 03 set of quantum chemistry codes. Calculations were performed at the Hartree-Fock (HF) and density functional theory (DFT) levels
Astrid Goubet et al.
Bioorganic & medicinal chemistry letters, 23(3), 761-763 (2012-12-26)
C5-Ethynylbenzenesulfonamide-modified nucleotide (EBNA) was investigated as substrate of various DNA polymerases. The experiments revealed that KOD, Phusion and Klenow DNA polymerases successfully accepted EBNA-T nucleotide as a substrate and yielded the fully extended DNA. KOD DNA polymerase was found to
Theres Ramenda et al.
Amino acids, 44(4), 1167-1180 (2013-01-12)
Cu(I)-mediated [3+2]cycloaddition between azides and alkynes has evolved into a valuable bioconjugation tool in radiopharmaceutical chemistry. We have developed a simple, convenient and reliable radiosynthesis of 4-[18F]fluoro-N-methyl-N-(propyl-2-yn-1-yl)benzenesulfonamide ([18F]F-SA) as a novel aromatic sulfonamide-based click chemistry building block. [18F]F-SA could be
Nitrogen-15 nuclear magnetic resonance study of benzenesulfonamide and cyanate binding to carbonic anhydrase.
K Kanamori et al.
Biochemistry, 22(11), 2658-2664 (1983-05-24)
Yousuke Takaoka et al.
Chemical communications (Cambridge, England), 49(27), 2801-2803 (2013-02-27)
Here we describe how a (19)F-probe incorporated into an endogenous protein by a chemical biology method revealed protein dynamics. By explicit determination of ligand-bound and unbound structures with X-ray crystallography, the quantitative comparison of the protein's dynamics in live cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)