Sulfaquinoxaline, product 1635206, has not been tested to be suitable for HPLC-MS-MS. Product 1635206 is used as a USP Reference Standard (no purity listed).
1635206
USP
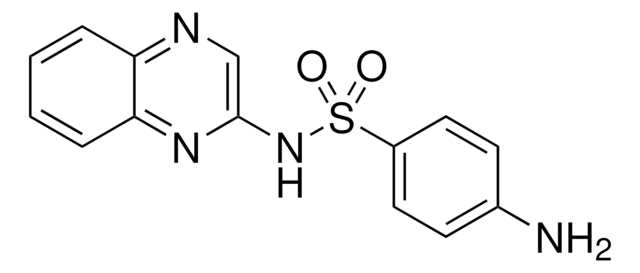
Sulfaquinoxaline
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
4-Amino-N-(2-quinoxalinyl)benzenesulfonamide, Sulfaquinoxalin
Select a Size
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
sulfaquinoxaline
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
Nc1ccc(cc1)S(=O)(=O)Nc2cnc3ccccc3n2
InChI
1S/C14H12N4O2S/c15-10-5-7-11(8-6-10)21(19,20)18-14-9-16-12-3-1-2-4-13(12)17-14/h1-9H,15H2,(H,17,18)
InChI key
NHZLNPMOSADWGC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Sulfaquinoxaline Oral Solution
Analysis Note
Other Notes
Related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
-
Good morning. I would like to know if this standard is suitable for use in hplc-ms-ms and the degree of purity of the analyte
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service