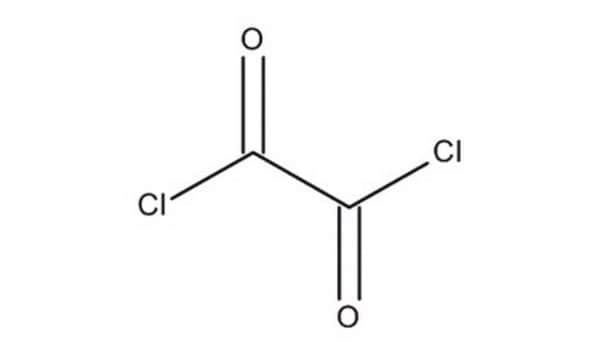
O8801
Oxalyl chloride
reagent grade, 98%
Synonym(s):
Ethanedioyl dichloride
About This Item
Recommended Products
grade
reagent grade
Quality Level
vapor density
4.4 (vs air)
vapor pressure
150 mmHg ( 20 °C)
assay
98%
reaction suitability
reagent type: oxidant
impurities
≤1.0% phosgene content
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
SMILES string
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To synthesize β, β′-diketodithioethers from β, β′-dihydroxydithioethers via Swern oxidation.
- In the DMSO-catalyzed Swern oxidation of primary amides or aldoximes to nitriles in the presence of triethylamine as a base.
- In the Moffatt-Swern oxidation of aryl allylic alcohols to halogenated unsaturated ketones in the presence of triethylamine.
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - Water-react 1
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service