522864
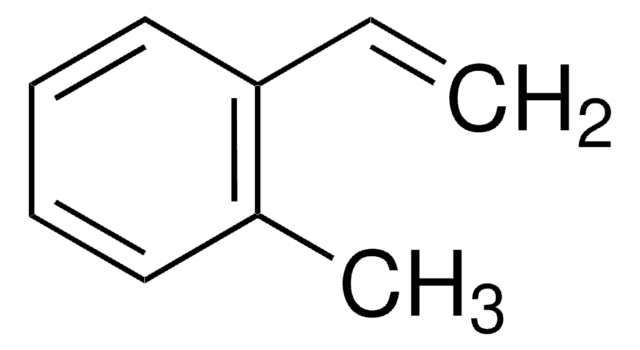
Methylstyrene
60% meta, 40% para and 1% ortho, 99%, contains ~50 ppm 4-tert-butylcatechol as inhibitor
Synonym(s):
Vinyltoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C6H4CH=CH2
CAS Number:
Molecular Weight:
118.18
Beilstein/REAXYS Number:
1209317
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
99%
contains
~50 ppm 4-tert-butylcatechol as inhibitor
refractive index
n20/D 1.5425 (lit.)
bp
168 °C (lit.)
density
0.893 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1ccc(C=C)cc1.Cc2cccc(C=C)c2
InChI
1S/2C9H10/c1-3-9-6-4-8(2)5-7-9;1-3-9-6-4-5-8(2)7-9/h2*3-7H,1H2,2H3
InChI key
VAPKHDZBJXRVNG-UHFFFAOYSA-N
Related Categories
General description
Methylstyrene can be used as a monomer in the synthesis of block copolymers for resins and adhesives. It is widely used as a starting material to prepare latexes for paper coating applications.
Application
- Applications in cancer therapy: Investigation into (−)-Epicatechin incorporated in phytopharmaceuticals for cancer treatment discusses its bioactive properties and synergistic effects when combined with other therapeutic agents, providing new avenues for integrative cancer therapies (Idoudi et al., 2024).
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
125.6 °F - closed cup
flash_point_c
52 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jihoon Kang et al.
Journal of the American Chemical Society, 130(37), 12273-12275 (2008-08-30)
A comprehensive structural and electrical characterization of solution-processed blend films of 6,13-bis(triisopropylsilylethynyl) pentacene (TIPS-pentacene) semiconductor and poly(alpha-methylstyrene) (PalphaMS) insulator was performed to understand and optimize the blend semiconductor films, which are very attractive as the active layer in solution-processed organic
Birgit Schiøtt et al.
Journal of the American Chemical Society, 124(49), 14558-14570 (2002-12-06)
Molecular dynamics simulations have been performed to gain insights into the catalytic mechanism of the hydrolysis of epoxides to vicinal diols by soluble epoxide hydrolase (sEH). The binding of a substrate, 1S,2S-trans-methylstyrene oxide, was studied in two conformations in the
Christine H Petter et al.
Journal of separation science, 31(14), 2541-2550 (2008-08-12)
The design of novel stationary phases is a permanent demanding challenge in chromatographic separation science to enable analysis with enhanced selectivity, specificity and speed. Therefore, the characterisation of chemical and physical properties is next to calculation of chromatographic parameters essential.
Sequence-Controlled alpha-Methylstyrene/Styrene Copolymers: Syntheses and Sequence Distribution Resolution
Arnaud Wolf, et al.
Macromolecules, 53, 8032-8040 (2020)
Xiang Zhang et al.
Rapid communications in mass spectrometry : RCM, 20(12), 1877-1882 (2006-05-24)
The interaction of the nitroxide radical traps (Tempo and Dmpo) and radicals produced in the electrophilic fluorination of olefins (styrene and alpha-methylstyrene) and Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octanebis(tetrafluoroborate) (F-TEDA-BF(4)) (1)) was investigated by electrospray ionization mass spectrometry (ESI-MS). Tempo succeeded in intercepting the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service