General description
Histone H3.1t (UniProt: Q16695; also known as H3/t, H3t, H3/g) is encoded by the HIST3H3 (also known as H3FT) gene (Gene ID: 8290) in human. Histones are highly conserved basic nuclear proteins that are responsible for the nucleosome structure of chromatin in eukaryotes. They play a central role in transcription regulation, DNA repair, DNA replication and chromosomal stability. Two molecules of each of the four core histones (H2A, H2B, H3, and H4) form an octamer, around which DNA is wrapped in repeating units, called nucleosomes, which limits DNA accessibility to the cellular machineries that require DNA as a template. Histone H3 features a main globular domain and a long N-terminal tail, which protrudes from the globular nucleosome core and can undergo several different types of epigenetic modifications that influence cellular processes. The N-terminal tails of histones are subject to posttranslational modifications, including phosphorylation, acetylation, and methylation, which recruit downstream regulatory factors, influence chromatin structure, and are critical determinants of transcription. Histone H3 can undergo acetylation on several lysine residues in the histone tail by histone acetyltransferases. Acetylation is generally associated with transcriptional activity and methylation of lysine and arginine residues can either activate or repress depending on the residue modified. Trimethylation of histone H3 is one of the most studied epigenetic marks. H3K4me3 modifications are reported to occur consistently at transcription start sites and H3K4me3 domain is associated with higher transcription activity and cell identity in pre-implantation development and in the process of deriving embryonic stem cells from the inner cell mass and trophoblast stem cells from the trophectoderm. Histone H3 is phosphorylated at threonine 4 (H3T3ph) by histone H3 associated protein kinase (HASPIN) during prophase and is dephosphorylated during anaphase and phosphorylation at serine 11 (H3S10ph) by Aurora kinase B is crucial for chromosome condensation and cell-cycle progression during mitosis and meiosis. (Ref.: Sharifi-Zarchi, A., et al. (2017). BMS Genomics. 18; Article 964; Liu, X., et al. (2016). Nature. 537(7621); 558-562).
Specificity
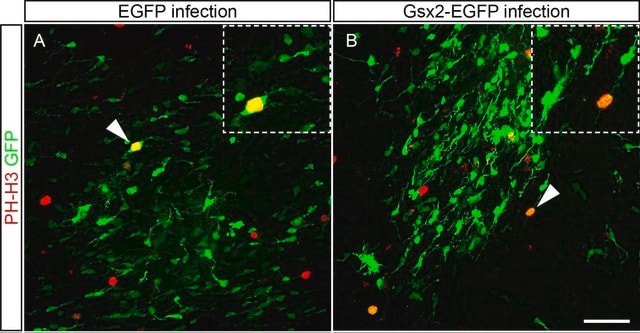
This rabbit polyclonal antibody detects Histone H3 phosphorylated on threonine 3.
Immunogen
KLH-conjugated linear peptide corresponding to 13 amino acids surrounding phosphothreonine 3 of human Histone H3.
Application
Quality Control Testing
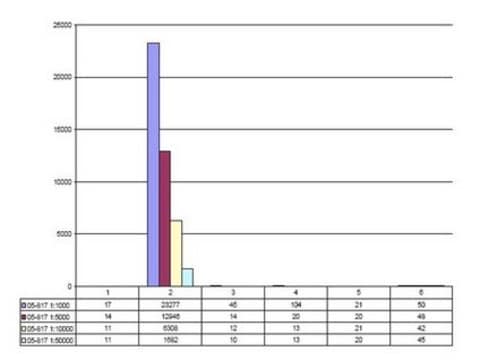
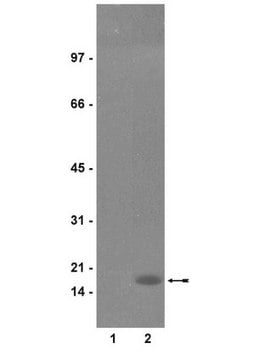
Evaluated by Western Blotting in acid extract from colcemid-treated HeLa cells.Western Blotting Analysis: A 1:5,000 dilution of this antibody detected Phospho-Histone H3 (Thr3) in acid extract of colcemid treated (50 ng/mL, overnight) HeLa cells, but not the recombinant Histone H3 protein.
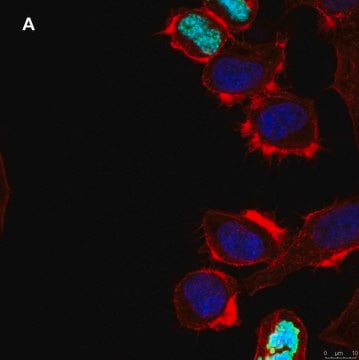
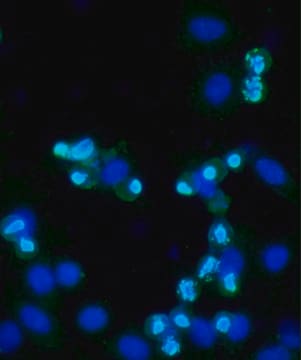
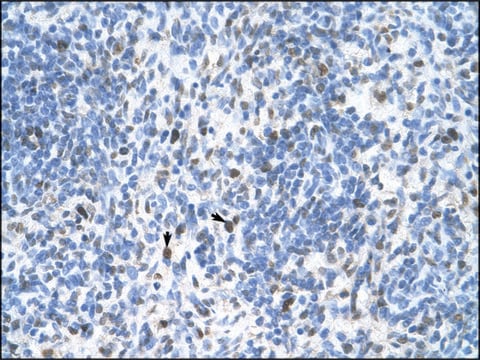
Tested ApplicationsPeptide Inhibition Assay: Target band detection in acid extract from colcemid treated (50 ng/mL; overnight) HeLa cells was prevented by preblocking of a representative lot with the immunogen phosphopeptide, but not with non-phosphorylated Histone H3 peptide.Immunocytochemistry Analysis: A 1:500 dilution from a representative lot detected Phospho-Histone H3 (Thr3) in NIH3T3 cells.Note: Actual optimal working dilutions must be determined by end user as specimens, and experimental conditions may vary with the end user.
Quality
routinely evaluated by immunoblot in acid extracted proteins from mitotic HeLa cells
Target description
~17 kDa observed; 15.51 kDa calculated. Uncharacterized bands may be observed in some lysate(s).
Physical form
Format: Unpurified
Immunodepleted
Immunodepleted rabbit serum with 0.05% sodium azide with 30% glycerol.
Storage and Stability
Store at -10°C to -25°C. Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Analysis Note
Control
Acid extract from colcemid-treated HeLa cells
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.