推薦產品
等級
pharmaceutical primary standard
API 家族
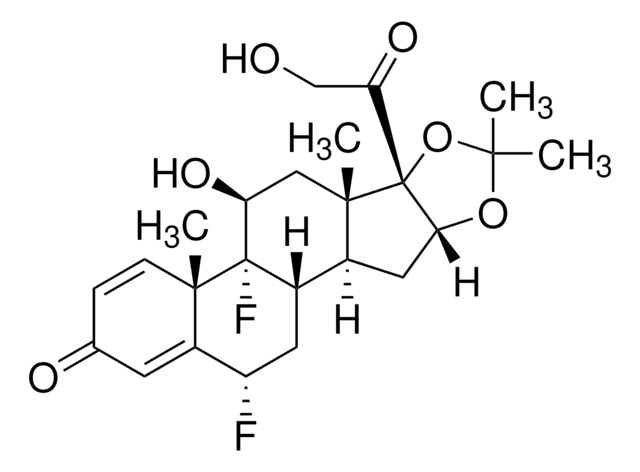
fluocinolone
製造商/商標名
USP
mp
267-269 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
CC1(C)O[C@@H]2C[C@H]3[C@@H]4C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO
InChI
1S/C24H30F2O6/c1-20(2)31-19-9-13-14-8-16(25)15-7-12(28)5-6-21(15,3)23(14,26)17(29)10-22(13,4)24(19,32-20)18(30)11-27/h5-7,13-14,16-17,19,27,29H,8-11H2,1-4H3/t13-,14-,16-,17-,19+,21-,22-,23-,24+/m0/s1
InChI 密鑰
FEBLZLNTKCEFIT-VSXGLTOVSA-N
基因資訊
human ... NR3C1(2908)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Fluocinolone acetonide USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Fluocinolone Acetonide Cream
- Fluocinolone Acetonide Ointment
- Fluocinolone Acetonide Topical Solution
- Neomycin Sulfate and Fluocinolone Acetonide Cream
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
R A Kenley et al.
Pharmaceutical research, 4(4), 342-347 (1987-08-01)
We investigated the degradation of fluocinolone acetonide (FA) incorporated into an oil-in-water cream base. The study examined the influence of temperature (23 to 80 degrees C) and cream pH (pH 2.3 to 6) on FA degradation rates. FA degradation followed
Elizabeth A Sugar et al.
Ophthalmology, 121(10), 1855-1862 (2014-06-09)
To evaluate the 3-year incremental cost-effectiveness of fluocinolone acetonide implant versus systemic therapy for the treatment of noninfectious intermediate, posterior, and panuveitis. Randomized, controlled, clinical trial. Patients with active or recently active intermediate, posterior, or panuveitis enrolled in the Multicenter
Wyatt B Messenger et al.
Drug design, development and therapy, 7, 425-434 (2013-06-06)
Diabetic macular edema (DME) remains one of the leading causes of moderate to severe vision loss. Although laser photocoagulation was the standard of care for several years, few patients achieved significant improvements in visual acuity. As a result, several pharmacotherapies
Reply: To PMID 24875002.
Trucian A Ostheimer et al.
American journal of ophthalmology, 159(2), 409-410 (2014-12-06)
Lea T Drye et al.
Clinical trials (London, England), 11(6), 635-647 (2014-08-15)
Investigators may elect to extend follow-up of participants enrolled in a randomized clinical trial after the trial comes to its planned end. The additional follow-up may be initiated to learn about longer term effects of treatments, including adverse events, costs
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務