推薦產品
化驗
≥97%
形狀
powder
光學活性
[α]25/D 149 to 161 °, c = 0.76% (w/v) in water
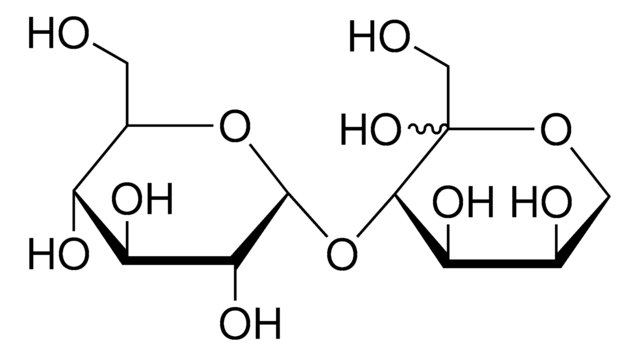
SMILES 字串
OC[C@H]1O[C@H](OC[C@H]2O[C@H](O[C@H]3[C@H](O)[C@@H](O)[C@@H](O)O[C@@H]3CO)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H32O16/c19-1-4-7(21)9(23)13(27)17(32-4)30-3-6-8(22)10(24)14(28)18(33-6)34-15-5(2-20)31-16(29)12(26)11(15)25/h4-29H,1-3H2/t4-,5-,6-,7-,8-,9+,10+,11-,12-,13-,14-,15-,16+,17+,18-/m1/s1
InChI 密鑰
OWEGMIWEEQEYGQ-QNHQVNOCSA-N
尋找類似的產品? 前往 產品比較指南
應用
D-Panose has been used in a study to determine the composition and sequence of glucan containing mixed linkages by carbon-13 nuclear magnetic resonance. It has also been used in a study to characterize electrophoretic behavior of sugar isomers.
生化/生理作用
Gluco-oligosaccharide consisting of three glucose residues.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
D Su et al.
Carbohydrate research, 248, 339-348 (1993-10-04)
In the maltose-acceptor reaction of Leuconostoc mesenteroides B-512FM dextransucrase, some of the D-glucose moieties of sucrose are diverted from the synthesis of dextran and are transferred to the nonreducing end of maltose to form panose. Glucose is also transferred to
Fabiano A N Fernandes et al.
Applied biochemistry and biotechnology, 142(1), 95-104 (2007-11-21)
Panose is a trisaccharide constituted by a maltose molecule bonded to a glucose molecule by an alpha-1,6-glycosidic bond. This trisaccharide has potential to be used in the food industry as a noncariogenic sweetener, as the oral flora does not ferment
G A Jeffrey et al.
Carbohydrate research, 222, 47-55 (1991-12-30)
The crystal structure of panose, O-alpha-D-glucopyranosyl-(1----6)-O-alpha-D-glucopyranosyl-(1----4)-D-gl ucose, C18H32O16, has been refined using low-temperature, 123 K, CuK alpha X-ray data. Difference syntheses and least-squares refinement showed a 16% substitution of alpha-panose by the beta anomer. All the hydrogen atoms were located
Manabu Sugimoto et al.
Bioscience, biotechnology, and biochemistry, 67(5), 1160-1163 (2003-07-02)
Transglucosylation activities of spinach alpha-glucosidase I and IV, which have different substrate specificity for hydrolyzing activity, were investigated. In a maltose mixture, alpha-glucosidase I, which has high activity toward not only maltooligosaccharides but also soluble starch and can hydrolyze isomaltose
T Kuriki et al.
Journal of bacteriology, 170(4), 1554-1559 (1988-04-01)
A new type of pullulanase which mainly produced panose from pullulan was found in Bacillus stearothermophilus and purified. The enzyme can hydrolyze pullulan efficiently and only hydrolyzes a small amount of starch. When pullulan was used as a substrate, the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務