推薦產品
化驗
≥98% (HPLC)
形狀
solid
溶解度
DMSO: 36.4 mg/mL
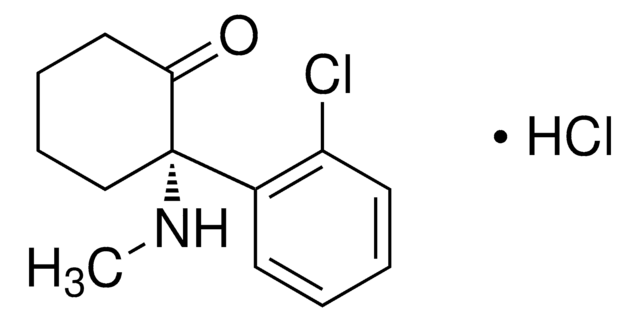
SMILES 字串
OC1=C(C(=O)Nc2cc(Cl)ccc12)c3cccc(Oc4ccccc4)c3
InChI
1S/C21H14ClNO3/c22-14-9-10-17-18(12-14)23-21(25)19(20(17)24)13-5-4-8-16(11-13)26-15-6-2-1-3-7-15/h1-12H,(H2,23,24,25)
InChI 密鑰
FLVRDMUHUXVRET-UHFFFAOYSA-N
基因資訊
human ... GRIN1(2902)
rat ... Grin1(24408) , Grin2a(24409)
生化/生理作用
L-701,324 is a high affinity, selective antagonist at the glycine site of the NMDA glutamate receptor. L-701,324 is a potent, active anticonvulsant with a reduced propensity to activate mesolimbic dopaminergic systems in rodents.
特點和優勢
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (Ion Channel Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
法律資訊
Manufactured and sold under license from Merck & Co., Inc., Kenilworth, NJ, U.S. U.S. Patent No. 5,348,962.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Kenneth W Perry et al.
Neuropharmacology, 55(5), 743-754 (2008-07-08)
Selective inhibitors of the glycine transporter 1 (GlyT1) have been implicated in central nervous system disorders related to hypoglutamatergic function such as schizophrenia. The selective GlyT1 inhibitors ALX5407 (NFPS) and LY2365109 {[2-(4-benzo[1,3]dioxol-5-yl-2-tert-butylphenoxy)ethyl]-methylamino}-acetic acid increased cerebrospinal fluid levels of glycine and
Anton Bespalov et al.
Behavioural pharmacology, 17(4), 295-302 (2006-08-18)
Previous studies suggested that adenosine A1 and A2A receptor agonists counteract behavioral effects of N-methyl-D-aspartate (NMDA) receptor antagonists while adenosine receptor antagonists may produce opposite effects enhancing the actions of NMDA receptor antagonists. To further evaluate the effects of combined
Jennifer E Murray et al.
Psychopharmacology, 213(1), 131-141 (2010-09-23)
Research using a drug discriminated goal-tracking (DGT) task showed that the N-methyl-D: -aspartate (NMDA) channel blocker MK-801 (dizocilpine) reduced the nicotine-evoked conditioned response (CR). Given the unknown mechanism of the effect, Experiment 1 replicated the MK-801 results and included tests
Kumar V S Nemmani et al.
Pain, 109(3), 274-283 (2004-05-26)
Pharmacological blockade of N-methyl-D-aspartate (NMDA) receptors can modulate morphine analgesia in experimental animals and humans. However, this literature is highly inconsistent, with NMDA receptor antagonists variously shown to potentiate, attenuate or produce no effect on morphine analgesic magnitude. A number
Masanobu Yoshikawa et al.
European journal of pharmacology, 565(1-3), 89-97 (2007-03-27)
Although there is a variety of information concerning the effects of the N-methyl-D-aspartate (NMDA) receptor on opioid-induced antinociception at the spinal level, little is known about the effects at the supraspinal level. To clarify the role of the NMDA receptor
文章
DISCOVER Bioactive Small Molecules for Neuroscience
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務