推薦產品
化驗
≥98% (HPLC)
形狀
solid
顏色
white
溶解度
DMSO: soluble >20 mg/mL
H2O: insoluble
儲存溫度
2-8°C
SMILES 字串
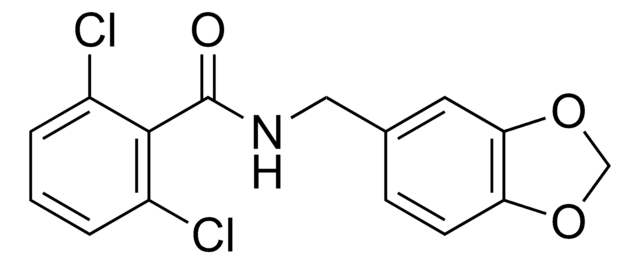
OC(=O)\C=C/C(O)=O.CCOC(=O)Nc1ccc(NCc2ccc(F)cc2)nc1N
InChI
1S/C15H17FN4O2.C4H4O4/c1-2-22-15(21)19-12-7-8-13(20-14(12)17)18-9-10-3-5-11(16)6-4-10;5-3(6)1-2-4(7)8/h3-8H,2,9H2,1H3,(H,19,21)(H3,17,18,20);1-2H,(H,5,6)(H,7,8)/b;2-1-
InChI 密鑰
DPYIXBFZUMCMJM-BTJKTKAUSA-N
生化/生理作用
Flupirtine is commercially available as maleate salt. It exists in two polymorphs : flupirtine maleate A and B. Flupirtine is useful in treating muscular spasm, muscle tension and muscle stiffness. It is known to be effective in relieving back pain. Along with cytoprotection, flupirtine also offers protection against neurodegenerative disorders such as multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer′s disease, Parkinson′s disease, Huntington′s chorea and AID (acquired immunodeficiency) associated encephalopathy. Flupirtine is found to be a potential drug for eye-related problems like maculopathy including diabetic retinopathy, retinitis pigmentosa and glaucoma. It is also proved to be helpful in preventing cardiac associated disorders such as myocardial ischemia and infarction, cerebral ischemia and infarction. Hepatitis is also prevented by the use of flupirtine.
Non-opioid analgesic; cytoprotective versus PrP fragment 106-126
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine.
Felicia Klinger et al.
British journal of pharmacology, 166(5), 1631-1642 (2011-12-23)
Flupirtine is a non-opioid analgesic that has been in clinical use for more than 20 years. It is characterized as a selective neuronal potassium channel opener (SNEPCO). Nevertheless, its mechanisms of action remain controversial and are the purpose of this
Anton Kolosov et al.
Pain medicine (Malden, Mass.), 13(11), 1444-1456 (2012-10-20)
Current treatments for cancer pain are often inadequate, particularly when metastasis to bone is involved. The addition to the treatment regimen of another drug that has a complementary analgesic effect may increase the overall analgesia without the necessity to increase
Fang Yu et al.
Epilepsy research, 97(1-2), 64-72 (2011-08-13)
Activation of KCNQ-channels has been shown to decrease or reduce the propagation of neuronal excitation in the immature central nervous system, and KCNQ activators represent a new class of anticonvulsant compounds. Their effectiveness has been demonstrated in many seizure models
Martin C Michel et al.
British journal of clinical pharmacology, 73(5), 821-825 (2011-11-03)
To determine efficacy of the analgesic flupirtine in the treatment of overactive bladder syndrome in a proof-of-concept study. Double-blind, double-dummy, three-armed comparison of flupirtine extended release (400 mg/day, titrated to 600 mg/day), tolterodine extended release (4 mg/day) and placebo for
Syed Mohammed Naser et al.
Journal of the Indian Medical Association, 110(3), 158-160 (2012-10-04)
The study was conducted to evaluate the efficacy and safety of flupirtine maleate 100 mg thrice daily compared to tramadol hydrochloride 50 mg thrice daily as postoperative pain management for 5 days. A total of 113 postoperative patients were recruited
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務