推薦產品
化驗
~96% (HPLC)
形狀
powder
儲存溫度
−20°C
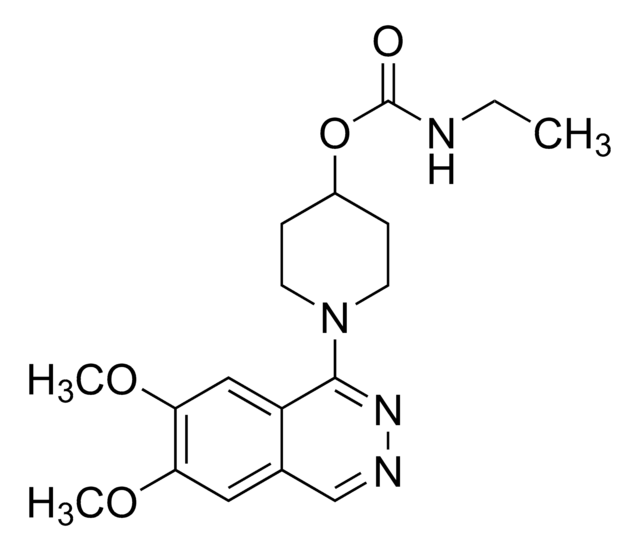
SMILES 字串
O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(O)=O)n2cnc3c(N[C@@H](CC(O)=O)C(O)=O)ncnc23
InChI
1S/C14H18N5O11P/c20-7(21)1-5(14(24)25)18-11-8-12(16-3-15-11)19(4-17-8)13-10(23)9(22)6(30-13)2-29-31(26,27)28/h3-6,9-10,13,22-23H,1-2H2,(H,20,21)(H,24,25)(H,15,16,18)(H2,26,27,28)/t5-,6+,9+,10+,13+/m0/s1
InChI 密鑰
OFBHPPMPBOJXRT-VWJPMABRSA-N
尋找類似的產品? 前往 產品比較指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
B Rees et al.
Journal of molecular biology, 299(5), 1157-1164 (2000-06-30)
The crystal structure of aspartyl-tRNA synthetase from Escherichia coli has been determined to a resolution of 2.7 A. The structure is compared to the same enzyme co-crystallized with tRNA(Asp) and containing aspartyl adenylate or ATP. The asymmetric unit contains three
H Mejdoub et al.
Biochemistry, 26(7), 2054-2059 (1987-04-07)
Aspartyl-tRNA synthetase from bakers' yeast gives an unstable complex with the cognate adenylate, which reacts after dissociation with amino acid side chains of the protein. This leads to a covalent incorporation of aspartic acid into aspartyl-tRNA synthetase via amide or
Maria Novikova et al.
The Journal of biological chemistry, 285(17), 12662-12669 (2010-02-18)
The heptapeptide-nucleotide microcin C (McC) is a potent inhibitor of enteric bacteria growth. McC is excreted from producing cells by the MccC transporter. The residual McC that remains in the producing cell can be processed by cellular aminopeptidases with the
Iu N Zhukov et al.
Bioorganicheskaia khimiia, 14(7), 969-972 (1988-07-01)
A number of earlier unknown phosphonate analogues of aspartyl adenylate with anhydride oxygen substituted by --CH2--, and the carbonyl group substituted by --CH(OH)- or --CH(NH2)-groups were synthesized. These compounds were used to study the reaction mechanism of asparagine synthetases from
Dominic Bernard et al.
Journal of enzyme inhibition and medicinal chemistry, 22(1), 77-82 (2007-03-22)
Asparaginyl-tRNA formation in Pseudomonas aeruginosa PAO1 involves a nondiscriminating aspartyl-tRNA synthetase (ND-AspRS) which forms Asp-tRNA(Asp) and Asp-tRNA(Asn), and a tRNA-dependent amidotransferase which transamidates the latter into Asn-tRNA(Asn). We report here that the inhibition of this ND-AspRS by L-aspartol adenylate (Asp-ol-AMP)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務