推薦產品
等級
pharmaceutical primary standard
API 家族
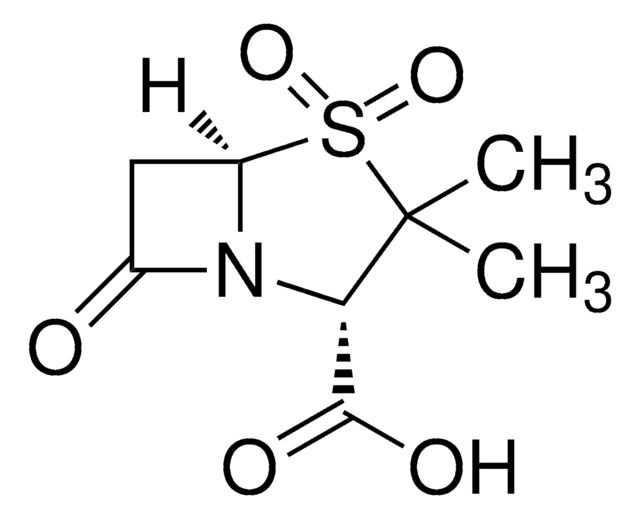
ceftazidime
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27;;;;;/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34);5*1H2/b26-13-;;;;;/t14-,18-;;;;;/m1...../s1
InChI 密鑰
NMVPEQXCMGEDNH-TZVUEUGBSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ceftazidime for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jingshu Ji et al.
Antimicrobial agents and chemotherapy, 57(11), 5697-5700 (2013-08-14)
It is unclear whether the genetic background of drug-resistant Pseudomonas aeruginosa was disseminated from a certain clone. Thus, we performed MLST (multilocus sequence typing) of 896 P. aeruginosa isolates that were nonsusceptible to imipenem, meropenem, or ceftazidime. This revealed 254
María M Tavío et al.
Journal of medical microbiology, 63(Pt 1), 56-65 (2013-10-04)
The mechanisms responsible for the increase in ceftazidime MIC in two Escherichia coli in vitro selected mutants, Caz/20-1 and Caz/20-2, were studied. OmpF loss and overexpression of acrB, acrD and acrF that were associated with acrR and marR mutations and
Covalent trapping and bacterial resistance to ceftazidime.
Jean-Marie A Frère
The Journal of biological chemistry, 288(37), 26967-26967 (2013-09-17)
Ayman M Goudah et al.
Journal of the American Veterinary Medical Association, 243(3), 424-429 (2013-07-20)
To determine the plasma disposition kinetics, absolute bioavailability, and milk concentrations of ceftazidime in healthy lactating female dromedary camels (Camelus dromedarius) following IV and IM administration of a single dose of 10 mg/kg (4.5 mg/lb). Prospective crossover study. 8 healthy
Jean-Marie Conil et al.
Clinical therapeutics, 35(10), 1603-1612 (2013-10-08)
Ceftazidime dosage regimen recommendations based on pharmacokinetic/pharmacodynamic approaches are not available for burn patients. The goal of this study was to propose a continuous dosage regimen of ceftazidime in burn patients, taking into account different MICs and pharmacokinetic covariates. The
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務